Comparison of Accelerated and Standard Hepatitis B Vaccination Schedules in High-Risk Healthy Adults: A Meta-Analysis of Randomized Controlled Trials
- PMID: 26196903
- PMCID: PMC4510064
- DOI: 10.1371/journal.pone.0133464
Comparison of Accelerated and Standard Hepatitis B Vaccination Schedules in High-Risk Healthy Adults: A Meta-Analysis of Randomized Controlled Trials
Abstract
Background: Selecting the most efficient vaccination schedule is an important issue.
Objective: To assess the beneficial and harmful effects of accelerated hepatitis B vaccination schedules in high-risk healthy adults.
Methods: We searched controlled trial registers of The Cochrane Library as well as MEDLINE, EMBASE, VIP Database for Chinese Technical Periodicals, and the Chinese National Knowledge Infrastructure databases for randomized controlled trials published up to December 2013 that compared accelerated hepatitis B vaccine schedules to the standard schedule in adults. The results were presented as relative risk with 95% confidence intervals. Fixed or random effect models were used for analysis.
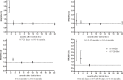
Results: We identified 10 randomized trials, all with one or more methodological weaknesses. Compared to the standard schedule, most accelerated schedules resulted in higher proportions of healthy vaccines more rapidly reaching anti-hepatitis B antibody levels >10 IU/L (P<0.05) initially and maintaining similar seroprotection rates after 6 months (P>0.05). Although accelerated schedules produced anti-hepatitis B levels higher than the standard schedule for the first month after the initial vaccine dose, they were significantly lower than the standard schedule after 6 months, except for an accelerated schedule that called for a fourth booster injection 12 months after the initial dose. Subjects administered accelerated vaccine schedules had similar compliance rate as those administered the standard schedule over the first 6 months of vaccination (relative risk = 1.00, 95% confidence interval: 0.84-1.21).
Conclusion: For rapid seroconversion and almost immediate short-term protection, accelerated vaccination schedules could be useful for at-risk groups. However, additional studies on the long-term protection and effectiveness of the primary doses of accelerated schedules are necessary.
Conflict of interest statement
Figures





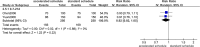

Similar articles
-
Schedules for hepatitis B vaccination of risk groups: balancing immunogenicity and compliance.Sex Transm Infect. 2007 Oct;83(6):426-32. doi: 10.1136/sti.2006.022111. Sex Transm Infect. 2007. PMID: 17911142 Free PMC article. Review.
-
Short-term immunogenicity of standard and accelerated hepatitis B virus vaccination schedules in healthy adults: a comparative field study in China.Biosci Rep. 2018 Oct 17;38(5):BSR20180846. doi: 10.1042/BSR20180846. Print 2018 Oct 31. Biosci Rep. 2018. PMID: 30201691 Free PMC article.
-
Immunogenicity of an accelerated vaccination regime with a combined hepatitis a/b vaccine in patients with chronic hepatitis C.Z Gastroenterol. 2003 Oct;41(10):983-90. doi: 10.1055/s-2003-42929. Z Gastroenterol. 2003. PMID: 14562195 Clinical Trial.
-
Comparison of accelerated and rapid schedules for monovalent hepatitis B and combined hepatitis A/B vaccines in children with cancer.Pediatr Hematol Oncol. 2007 Dec;24(8):587-94. doi: 10.1080/08880010701703511. Pediatr Hematol Oncol. 2007. PMID: 18092249 Clinical Trial.
-
The place of accelerated schedules for hepatitis A and B vaccinations.Drugs. 2003;63(17):1779-84. doi: 10.2165/00003495-200363170-00001. Drugs. 2003. PMID: 12921484 Review.
Cited by
-
Hepatitis Vaccines.Vaccines (Basel). 2016 Mar 11;4(1):6. doi: 10.3390/vaccines4010006. Vaccines (Basel). 2016. PMID: 26978406 Free PMC article. Review.
-
Schistosoma mansoni Infection Can Jeopardize the Duration of Protective Levels of Antibody Responses to Immunizations against Hepatitis B and Tetanus Toxoid.PLoS Negl Trop Dis. 2016 Dec 7;10(12):e0005180. doi: 10.1371/journal.pntd.0005180. eCollection 2016 Dec. PLoS Negl Trop Dis. 2016. PMID: 27926921 Free PMC article.
-
Comparison of Serological Immune Response to Hepatitis B Vaccine Following Rapid or Standard Regimen in People Who Inject Drugs.J Clin Exp Hepatol. 2025 May-Jun;15(3):102501. doi: 10.1016/j.jceh.2025.102501. Epub 2025 Jan 9. J Clin Exp Hepatol. 2025. PMID: 39975859
-
A Randomized Study Comparing the Efficacy of Three Hepatitis B Vaccine Induction Regimens in Adult Patients with Hematological Malignancies.Turk J Haematol. 2016 Sep 5;33(3):231-5. doi: 10.4274/tjh.2015.0079. Epub 2016 Apr 18. Turk J Haematol. 2016. PMID: 27094506 Free PMC article. Clinical Trial.
-
A Global View to HBV Chronic Infection: Evolving Strategies for Diagnosis, Treatment and Prevention in Immunocompetent Individuals.Int J Environ Res Public Health. 2019 Sep 9;16(18):3307. doi: 10.3390/ijerph16183307. Int J Environ Res Public Health. 2019. PMID: 31505743 Free PMC article. Review.
References
-
- Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. 2006. J Hepatol; 45(4):529–38. - PubMed
-
- Rich JD, Ching CG, Lally MA, Gaitanis MM, Schwartzapfel B, Charuvastra A, et al. A review of the case for hepatitis B vaccination of high-risk adults. Am J Med. 2003; 114(4):316–8. - PubMed
-
- Goldstein ST, Alter MJ, Williams IT, Moyer LA, Judson FN, Mottram K, et al. Incidence and risk factors for acute hepatitis B in the United States, 1982–1998: implications for vaccination programs. J Infect Dis. 2002; 185(6):713–9. - PubMed
-
- Wahl M, Hermodsson S, Iwarson S. Hepatitis B vaccination with short dose intervals—a possible alternative for post-exposure prophylaxis? Infection. 1988; 16(4):229–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

