Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery
- PMID: 26197299
- PMCID: PMC4518285
- DOI: 10.1038/ncomms8715
Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery
Abstract
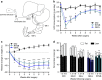
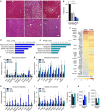
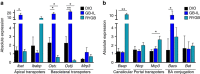
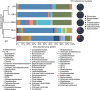
Roux-en-Y gastric bypass (RYGB) is highly effective in reversing obesity and associated diabetes. Recent observations in humans suggest a contributing role of increased circulating bile acids in mediating such effects. Here we use a diet-induced obesity (DIO) mouse model and compare metabolic remission when bile flow is diverted through a gallbladder anastomosis to jejunum, ileum or duodenum (sham control). We find that only bile diversion to the ileum results in physiologic changes similar to RYGB, including sustained improvements in weight, glucose tolerance and hepatic steatosis despite differential effects on hepatic gene expression. Circulating free fatty acids and triglycerides decrease while bile acids increase, particularly conjugated tauro-β-muricholic acid, an FXR antagonist. Activity of the hepatic FXR/FGF15 signalling axis is reduced and associated with altered gut microbiota. Thus bile diversion, independent of surgical rearrangement of the gastrointestinal tract, imparts significant weight loss accompanied by improved glucose and lipid homeostasis that are hallmarks of RYGB.
Figures




Comment in
-
Surgery: Bile diversion comparable to bariatric surgery in mice.Nat Rev Gastroenterol Hepatol. 2015 Sep;12(9):488. doi: 10.1038/nrgastro.2015.130. Epub 2015 Jul 28. Nat Rev Gastroenterol Hepatol. 2015. PMID: 26215388 No abstract available.
References
-
- Mingrone G. et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N. Engl. J. Med. 366, 1577–1585 (2012) . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T35 DK007383/DK/NIDDK NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- R01 DK091748/DK/NIDDK NIH HHS/United States
- R01 DK105847/DK/NIDDK NIH HHS/United States
- P30 CA68485/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- DK058404/DK/NIDDK NIH HHS/United States
- U2C DK092993/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- 5UL1 RR024975-03/RR/NCRR NIH HHS/United States
- U24 DK092993/DK/NIDDK NIH HHS/United States
- S10 OD016355/OD/NIH HHS/United States
- P30 EY08126/EY/NEI NIH HHS/United States
- DK059637/DK/NIDDK NIH HHS/United States
- G20 RR030956/RR/NCRR NIH HHS/United States
- DK020593/DK/NIDDK NIH HHS/United States
- U24-DK 092993/DK/NIDDK NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- R24 DK096527/DK/NIDDK NIH HHS/United States
- F32DK103474/DK/NIDDK NIH HHS/United States
- F32 DK103474/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

