Synthesis, spectroscopic investigations (X-ray, NMR and TD-DFT), antimicrobial activity and molecular docking of 2,6-bis(hydroxy(phenyl)methyl)cyclohexanone
- PMID: 26197312
- PMCID: PMC6332055
- DOI: 10.3390/molecules200713240
Synthesis, spectroscopic investigations (X-ray, NMR and TD-DFT), antimicrobial activity and molecular docking of 2,6-bis(hydroxy(phenyl)methyl)cyclohexanone
Abstract
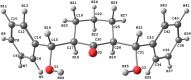
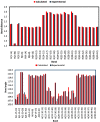
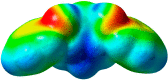
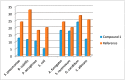
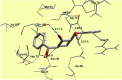
The synthesis of 2,6-bis(hydroxy(phenyl)methyl)cyclohexanone 1 is described. The molecular structure of the title compound 1 was confirmed by NMR, FT-IR, MS, CHN microanalysis, and X-ray crystallography. The molecular structure was also investigated by a set of computational studies and found to be in good agreement with the experimental data obtained from the various spectrophotometric techniques. The antimicrobial activity and molecular docking of the synthesized compound was investigated.
Keywords: Aldol product; DFT; TGA; X-ray; antimicrobial activity; cyclohexanone; molecular docking.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
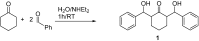










Similar articles
-
Synthesis, molecular docking studies, and antimicrobial evaluation of new structurally diverse ureas.Bioorg Chem. 2019 Jun;87:302-311. doi: 10.1016/j.bioorg.2019.03.024. Epub 2019 Mar 19. Bioorg Chem. 2019. PMID: 30913465
-
Hydrazonoyl Chlorides as Precursors for Synthesis of Novel Bis-Pyrrole Derivatives.Molecules. 2016 Mar 9;21(3):326. doi: 10.3390/molecules21030326. Molecules. 2016. PMID: 27005604 Free PMC article.
-
Synthesis, biological evaluation and molecular docking of some substituted pyrazolines and isoxazolines as potential antimicrobial agents.Eur J Med Chem. 2015 May 5;95:96-103. doi: 10.1016/j.ejmech.2015.03.031. Epub 2015 Mar 16. Eur J Med Chem. 2015. PMID: 25800645
-
Synthesis, characterization, and evaluation of (E)-methyl 2-((2-oxonaphthalen-1(2H)-ylidene)methylamino)acetate as a biological agent and an anion sensor.Bioorg Med Chem. 2016 Nov 1;24(21):5592-5601. doi: 10.1016/j.bmc.2016.09.018. Epub 2016 Sep 13. Bioorg Med Chem. 2016. PMID: 27658791
-
New polyfunctional imidazo[4,5-C]pyridine motifs: synthesis, crystal studies, docking studies and antimicrobial evaluation.Eur J Med Chem. 2014 Apr 22;77:288-97. doi: 10.1016/j.ejmech.2014.03.019. Epub 2014 Mar 11. Eur J Med Chem. 2014. PMID: 24657565
Cited by
-
Computational studies of 2-(4-oxo-3-phenylthiazolidin-2-ylidene)malononitrile.BMC Chem. 2019 Feb 18;13(1):25. doi: 10.1186/s13065-019-0542-6. eCollection 2019 Dec. BMC Chem. 2019. PMID: 31384774 Free PMC article.
References
-
- Mahrwald R. Modern Aldol Reactions. Volumes 1–2 Wiley-VCH; Weinheim, Germany: 2004.
-
- Artico M., Santo R.D., Costi R., Novellino E., Greco G., Massa S., Tramontano E., Marongiu M.E., Montis A.D., Colla P.L. Geometrically and conformationally restrained cinnamoyl compounds as inhibitors of HIV-1 integrase: Synthesis, biological evaluation, and molecular modeling. J. Med. Chem. 1998;41:3948–3960. doi: 10.1021/jm9707232. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

