Progress in corneal wound healing
- PMID: 26197361
- PMCID: PMC4651844
- DOI: 10.1016/j.preteyeres.2015.07.002
Progress in corneal wound healing
Abstract
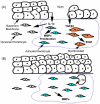
Corneal wound healing is a complex process involving cell death, migration, proliferation, differentiation, and extracellular matrix remodeling. Many similarities are observed in the healing processes of corneal epithelial, stromal and endothelial cells, as well as cell-specific differences. Corneal epithelial healing largely depends on limbal stem cells and remodeling of the basement membrane. During stromal healing, keratocytes get transformed to motile and contractile myofibroblasts largely due to activation of transforming growth factor-β (TGF-β) system. Endothelial cells heal mostly by migration and spreading, with cell proliferation playing a secondary role. In the last decade, many aspects of wound healing process in different parts of the cornea have been elucidated, and some new therapeutic approaches have emerged. The concept of limbal stem cells received rigorous experimental corroboration, with new markers uncovered and new treatment options including gene and microRNA therapy tested in experimental systems. Transplantation of limbal stem cell-enriched cultures for efficient re-epithelialization in stem cell deficiency and corneal injuries has become reality in clinical setting. Mediators and course of events during stromal healing have been detailed, and new treatment regimens including gene (decorin) and stem cell therapy for excessive healing have been designed. This is a very important advance given the popularity of various refractive surgeries entailing stromal wound healing. Successful surgical ways of replacing the diseased endothelium have been clinically tested, and new approaches to accelerate endothelial healing and suppress endothelial-mesenchymal transformation have been proposed including Rho kinase (ROCK) inhibitor eye drops and gene therapy to activate TGF-β inhibitor SMAD7. Promising new technologies with potential for corneal wound healing manipulation including microRNA, induced pluripotent stem cells to generate corneal epithelium, and nanocarriers for corneal drug delivery are discussed. Attention is also paid to problems in wound healing understanding and treatment, such as lack of specific epithelial stem cell markers, reliable identification of stem cells, efficient prevention of haze and stromal scar formation, lack of data on wound regulating microRNAs in keratocytes and endothelial cells, as well as virtual lack of targeted systems for drug and gene delivery to select corneal cells.
Keywords: Corneal endothelium; Corneal epithelium; Gene therapy; Keratocyte; Stem cell; Wound healing.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




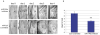
References
-
- Aberdam E, Barak E, Rouleau M, de LaForest S, Berrih-Aknin S, Suter DM, Krause KH, Amit M, Itskovitz-Eldor J, Aberdam D. A pure population of ectodermal cells derived from human embryonic stem cells. Stem Cells. 2008;26:440–444. - PubMed
-
- Abrams GA, Schaus SS, Goodman SL, Nealey PF, Murphy CJ. Nanoscale topography of the corneal epithelial basement membrane and Descemet’s membrane of the human. Cornea. 2000;19:57–64. - PubMed
-
- Ahmad S, Kolli S, Lako M, Figueiredo F, Daniels JT. Stem cell therapies for ocular surface disease. Drug Discov. Today. 2010;15:306–313. - PubMed
-
- Alio JL, Pastor S, Ruiz-Colecha J, Rodriguez A, Artola A. Treatment of ocular surface syndrome after LASIK with autologous platelet-rich plasma. J. Refract. Surg. 2007;23:617–619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

