Transglycosylation Activity of Glycosynthase Mutants of Endo-β-N-Acetylglucosaminidase from Coprinopsis cinerea
- PMID: 26197478
- PMCID: PMC4510386
- DOI: 10.1371/journal.pone.0132859
Transglycosylation Activity of Glycosynthase Mutants of Endo-β-N-Acetylglucosaminidase from Coprinopsis cinerea
Abstract
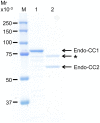
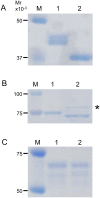
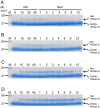
Endo-β-N-acetylglucosaminidase (ENGase), which catalyzes hydrolysis of N-linked oligosaccharides, is a useful tool for analyzing oligosaccharide contents of glycoproteins. However, there are only a few known ENGases that can catalyze the hydrolysis of human complex type oligosaccharides, and although commercially available, they are expensive. Here, we report the cloning of two ENGase encoding cDNAs from the basidiomycete fungus Coprinopsis cinerea, Endo-CC1 and Endo-CC2. We successfully expressed recombinant His6-tagged Endo-CC1 and Endo-CC2 in Escherichia coli and purified them for enzymatic characterization. Both Endo-CC1 and Endo-CC2 showed hydrolytic activity on high-mannose and complex type oligosaccharides. Since Endo-CC1 could be prepared more easily than Endo-CC2 from E. coli cultures, we examined the enzymatic properties of Endo-CC1 in detail. Our results showed that Endo-CC1 acted on both N-linked high-mannose type and sialobiantennary type complex oligosaccharides of glycoproteins RNase B and human transferrin, respectively, but not on the sialotriantennary type complex oligosaccharide of glycoprotein fetuin. Examination of the transglycosylation activity of Endo-CC1 revealed that the wild-type Endo-CC1 could not transfer the sialobiantennary type complex oligosaccharide onto the deglycosylated RNase B. To obtain an Endo-CC1 mutant with desired transglycosylation activity, we performed mutation analysis of the active site residue Asn 180 (N180), known to be important for catalysis, by individually replacing it with the remaining 19 amino acid residues. Transglycosylation analyses of these mutants led us to identify one mutant, namely Endo-CC1N180H, which exhibited the desired transglycosylation activity. Taken together, we suggest that Endo-CC1 would potentially be a valuable tool for analyzing oligosaccharides on glycoproteins, as large quantities of it could be made available more easily and less expensively than the currently used enzyme, Endo-M.
Conflict of interest statement
Figures





Similar articles
-
Molecular cloning of Mucor hiemalis endo-beta-N-acetylglucosaminidase and some properties of the recombinant enzyme.Arch Biochem Biophys. 2004 Dec 1;432(1):41-9. doi: 10.1016/j.abb.2004.09.013. Arch Biochem Biophys. 2004. PMID: 15519295
-
Highly efficient transglycosylation of sialo-complex-type oligosaccharide using Coprinopsis cinerea endoglycosidase and sugar oxazoline.Biotechnol Lett. 2017 Jan;39(1):157-162. doi: 10.1007/s10529-016-2230-0. Epub 2016 Oct 6. Biotechnol Lett. 2017. PMID: 27714557
-
Identification and characterization of endo-β-N-acetylglucosaminidase from methylotrophic yeast Ogataea minuta.Glycobiology. 2013 Jun;23(6):736-44. doi: 10.1093/glycob/cwt012. Epub 2013 Feb 22. Glycobiology. 2013. PMID: 23436287
-
Chemoenzymatic synthesis of glycopeptides and glycoproteins through endoglycosidase-catalyzed transglycosylation.Carbohydr Res. 2008 Jul 21;343(10-11):1509-22. doi: 10.1016/j.carres.2008.03.025. Epub 2008 Mar 27. Carbohydr Res. 2008. PMID: 18405887 Free PMC article. Review.
-
Microbial endoglycosidases for analyses of oligosaccharide chains in glycoproteins.J Biochem. 1994 Aug;116(2):229-35. doi: 10.1093/oxfordjournals.jbchem.a124510. J Biochem. 1994. PMID: 7822234 Review.
Cited by
-
Selective N-glycan editing on living cell surfaces to probe glycoconjugate function.Nat Chem Biol. 2020 Jul;16(7):766-775. doi: 10.1038/s41589-020-0551-8. Epub 2020 Jun 1. Nat Chem Biol. 2020. PMID: 32483376
-
Synthesis of Glycosides by Glycosynthases.Molecules. 2017 Aug 30;22(9):1434. doi: 10.3390/molecules22091434. Molecules. 2017. PMID: 28867807 Free PMC article. Review.
-
N-glycan-modified α-L-iduronidase produced by transgenic silkworms ameliorates clinical signs in a Japanese macaque with mucopolysaccharidosis I.Commun Med (Lond). 2025 Apr 18;5(1):128. doi: 10.1038/s43856-025-00841-7. Commun Med (Lond). 2025. PMID: 40251406 Free PMC article.
-
Site-Specific Glyco-Tagging of Native Proteins for the Development of Biologicals.J Am Chem Soc. 2024 Dec 18;146(50):34452-34465. doi: 10.1021/jacs.4c11091. Epub 2024 Dec 9. J Am Chem Soc. 2024. PMID: 39653378 Free PMC article.
-
Catanionic Vesicles as a Facile Scaffold to Display Natural N-Glycan Ligands for Probing Multivalent Carbohydrate-Lectin Interactions.Bioconjug Chem. 2023 Feb 15;34(2):392-404. doi: 10.1021/acs.bioconjchem.2c00560. Epub 2023 Jan 15. Bioconjug Chem. 2023. PMID: 36642983 Free PMC article.
References
-
- Helenius A, Aebi M (2001) Intracellular functions of N-linked glycans. Science 291: 2364–2369. - PubMed
-
- Yamaguchi Y, Nishimura M, Nagano M, Yagi H, Sasakawa H, Uchida K, et al. (2006) Glycoform-dependent conformational alteration of the Fc region of human immunoglobulin G1 as revealed by NMR spectroscopy. Biochim Biophys Acta 1760: 693–700. - PubMed
-
- Matsumiya S, Yamaguchi Y, Saito J, Nagano M, Sasakawa H, Otaki S, et al. (2007) Structural comparison of fucosylated and nonfucosylated Fc fragments of human immunoglobulin G1. J Mol Biol 368: 767–779. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

