Clinical significance of systematic retroperitoneal lymphadenectomy during interval debulking surgery in advanced ovarian cancer patients
- PMID: 26197771
- PMCID: PMC4620367
- DOI: 10.3802/jgo.2015.26.4.303
Clinical significance of systematic retroperitoneal lymphadenectomy during interval debulking surgery in advanced ovarian cancer patients
Abstract
Objective: To investigate the clinical significance of systematic retroperitoneal lymphadenectomy during interval debulking surgery (IDS) in advanced epithelial ovarian cancer (EOC) patients.
Methods: We retrospectively reviewed the medical records of 124 advanced EOC patients and analyzed the details of neoadjuvant chemotherapy (NACT), IDS, postoperative treatment, and prognoses.
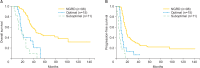
Results: Following IDS, 98 patients had no gross residual disease (NGRD), 15 had residual disease sized <1 cm (optimal), and 11 had residual disease sized ≥1 cm (suboptimal). Two-year overall survival (OS) and progression-free survival (PFS) rates were 88.8% and 39.8% in the NGRD group, 40.0% and 13.3% in the optimal group (p<0.001 vs. NGRD for both), and 36.3% and 0% in the suboptimal group, respectively. Five-year OS and 2-year PFS rates were 62% and 56.1% in the lymph node-negative (LN-) group and 26.2% and 24.5% in the lymph node-positive (LN+) group (p=0.0033 and p=0.0024 vs. LN-, respectively). Furthermore, survival in the LN+ group, despite surgical removal of positive nodes, was the same as that in the unknown LN status group, in which lymphadenectomy was not performed (p=0.616 and p=0.895, respectively). Multivariate analysis identified gross residual tumor during IDS (hazard ratio, 3.68; 95% confidence interval, 1.31 to 10.33 vs. NGRD) as the only independent predictor of poor OS.
Conclusion: NGRD after IDS improved prognosis in advanced EOC patients treated with NACT-IDS. However, while systematic retroperitoneal lymphadenectomy during IDS may predict outcome, it does not confer therapeutic benefits.
Keywords: Cytoreduction Surgical Procedures; Disease-Free Survival; Lymph Node Excision; Neoadjuvant Therapy; Neoplasm, Residual; Ovarian Neoplasms.
Conflict of interest statement
Figures

References
-
- Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S161–S192. - PubMed
-
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. - PubMed
-
- Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. - PubMed
-
- Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener HC, Lopes T, et al. Chemotherapy or upfront surgery for newly diagnosed advanced ovarian cancer: results from the MRC CHORUS trial. J Clin Oncol. 2013;31(15 Suppl):5500.
-
- Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–564. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

