Pharmacokinetics of intravenous sildenafil in children with palliated single ventricle heart defects: effect of elevated hepatic pressures
- PMID: 26197839
- PMCID: PMC4591210
- DOI: 10.1017/S1047951115000359
Pharmacokinetics of intravenous sildenafil in children with palliated single ventricle heart defects: effect of elevated hepatic pressures
Abstract
Aims Sildenafil is frequently prescribed to children with single ventricle heart defects. These children have unique hepatic physiology with elevated hepatic pressures, which may alter drug pharmacokinetics. We sought to determine the impact of hepatic pressure on sildenafil pharmacokinetics in children with single ventricle heart defects.
Methods: A population pharmacokinetic model was developed using data from 20 single ventricle children receiving single-dose intravenous sildenafil during cardiac catheterisation. Non-linear mixed effect modelling was used for model development, and covariate effects were evaluated based on estimated precision and clinical significance.
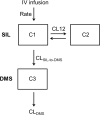
Results: The analysis included a median (range) of 4 (2-5) pharmacokinetic samples per child. The final structural model was a two-compartment model for sildenafil with a one-compartment model for des-methyl-sildenafil (active metabolite), with assumed 100% sildenafil to des-methyl-sildenafil conversion. Sildenafil clearance was unaffected by hepatic pressure (clearance=0.62 L/hour/kg); however, clearance of des-methyl-sildenafil (1.94×(hepatic pressure/9)(-1.33) L/hour/kg) was predicted to decrease ~7-fold as hepatic pressure increased from 4 to 18 mmHg. Predicted drug exposure was increased by ~1.5-fold in subjects with hepatic pressures ⩾10 versus <10 mmHg (median area under the curve=533 versus 792 µg*h/L). Discussion Elevated hepatic pressure delays clearance of the sildenafil metabolite - des-methyl-sildenafil - and increases drug exposure. We speculate that this results from impaired biliary clearance. Hepatic pressure should be considered when prescribing sildenafil to children. These data demonstrate the importance of pharmacokinetic assessments in patients with unique cardiovascular physiology that may affect drug metabolism.
Keywords: Single ventricle; hepatic dysfunction; pharmacokinetics; sildenafil.
Conflict of interest statement
None
Figures


References
-
- Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23(5):687–696. - PubMed
-
- Narkewicz MR, Sondheimer HM, Ziegler JW, et al. Hepatic dysfunction following the Fontan procedure. J Pediatr Gastroenterol Nutr. 2003;36(3):352–357. - PubMed
-
- Wu FM, Ukomadu C, Odze RD, et al. Earing MG. Liver disease in the patient with Fontan circulation. Congenit Heart Dis. 2011;6(3):190–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

