Randomized Trial of Rapid Multiplex Polymerase Chain Reaction-Based Blood Culture Identification and Susceptibility Testing
- PMID: 26197846
- PMCID: PMC4560903
- DOI: 10.1093/cid/civ447
Randomized Trial of Rapid Multiplex Polymerase Chain Reaction-Based Blood Culture Identification and Susceptibility Testing
Abstract
Background: The value of rapid, panel-based molecular diagnostics for positive blood culture bottles (BCBs) has not been rigorously assessed. We performed a prospective randomized controlled trial evaluating outcomes associated with rapid multiplex PCR (rmPCR) detection of bacteria, fungi, and resistance genes directly from positive BCBs.
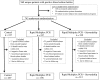
Methods: A total of 617 patients with positive BCBs underwent stratified randomization into 3 arms: standard BCB processing (control, n = 207), rmPCR reported with templated comments (rmPCR, n = 198), or rmPCR reported with templated comments and real-time audit and feedback of antimicrobial orders by an antimicrobial stewardship team (rmPCR/AS, n = 212). The primary outcome was antimicrobial therapy duration. Secondary outcomes were time to antimicrobial de-escalation or escalation, length of stay (LOS), mortality, and cost.
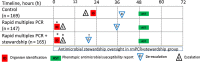
Results: Time from BCB Gram stain to microorganism identification was shorter in the intervention group (1.3 hours) vs control (22.3 hours) (P < .001). Compared to the control group, both intervention groups had decreased broad-spectrum piperacillin-tazobactam (control 56 hours, rmPCR 44 hours, rmPCR/AS 45 hours; P = .01) and increased narrow-spectrum β-lactam (control 42 hours, rmPCR 71 hours, rmPCR/AS 85 hours; P = .04) use, and less treatment of contaminants (control 25%, rmPCR 11%, rmPCR/AS 8%; P = .015). Time from Gram stain to appropriate antimicrobial de-escalation or escalation was shortest in the rmPCR/AS group (de-escalation: rmPCR/AS 21 hours, control 34 hours, rmPCR 38 hours, P < .001; escalation: rmPCR/AS 5 hours, control 24 hours, rmPCR 6 hours, P = .04). Groups did not differ in mortality, LOS, or cost.
Conclusions: rmPCR reported with templated comments reduced treatment of contaminants and use of broad-spectrum antimicrobials. Addition of antimicrobial stewardship enhanced antimicrobial de-escalation.
Clinical trials registration: NCT01898208.
Keywords: PCR; antimicrobial stewardship; blood culture; diagnostic.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Editorial Commentary: Rapid Blood Culture Identification: The Value of a Randomized Trial.Clin Infect Dis. 2015 Oct 1;61(7):1081-3. doi: 10.1093/cid/civ450. Epub 2015 Jul 20. Clin Infect Dis. 2015. PMID: 26197845 No abstract available.
-
Reply to Idelevich and Beck.Clin Infect Dis. 2016 Jan 15;62(2):269-70. doi: 10.1093/cid/civ826. Epub 2015 Sep 22. Clin Infect Dis. 2016. PMID: 26394668 No abstract available.
-
Identification and Susceptibility Testing From Shortly Incubated Cultures Accelerate Blood Culture Diagnostics at No Cost.Clin Infect Dis. 2016 Jan 15;62(2):268-9. doi: 10.1093/cid/civ824. Epub 2015 Sep 22. Clin Infect Dis. 2016. PMID: 26394670 No abstract available.
References
-
- Ibrahim E, Sherman G, Ward S, Fraser V, Kollef M. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000; 118:46–55. - PubMed
-
- Garnacho-Montero J, Gutierrez-Pizarraya A, Escoresca-Ortega A et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med 2014; 40:32–40. - PubMed
-
- Dellit T, Owens R, McGowan J et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an an institutional program for enhancing antimicrobial stewardship. Clin Infect Dis 2007; 44:159–77. - PubMed
-
- Perez K, Olsen R, Musick W et al. Integrating rapid pathogen identification and antimicrobial stewardship significantly decreases hospital costs. Arch Pathol Lab Med 2012; 137:1247–54. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

