Platinum sensitivity and non-cross-resistance of cisplatin analogue with cisplatin in recurrent cervical cancer
- PMID: 26197856
- PMCID: PMC4510334
- DOI: 10.3802/jgo.2015.26.3.185
Platinum sensitivity and non-cross-resistance of cisplatin analogue with cisplatin in recurrent cervical cancer
Abstract
Objective: The concept of platinum sensitivity and cross-resistance among platinum agents are widely known in the management of recurrent ovarian cancer. The aim of this study was to evaluate two hypotheses regarding the validity of the concept of platinum sensitivity and non-cross-resistance of cisplatin analogue with cisplatin in recurrent cervical cancer.
Methods: In this retrospective study, the clinical data of patients with recurrent cervical cancer, who had a history of receiving cisplatin based chemotherapy (including concurrent chemoradiotherapy [CCRT] with cisplatin) and who received second-line chemotherapy at the time of recurrence between April 2004 and July 2012 were reviewed.
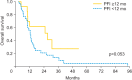
Results: In total, 49 patients--34 squamous cell carcinomas (69.4%) and 15 non-squamous cell carcinomas (30.6%)--were enrolled. The median age was 53 years (range, 26 to 79 years). Univariate and multivariate analysis showed that a platinum free interval (PFI) of 12 months has a strong relationship with the response rate to second-line chemotherapy. Upon multivariate analysis of survival after second-line platinum-based chemotherapy, a PFI of 12 months significantly influenced both progression-free survival (hazard ratio [HR], 0.349; 95% confidence interval [CI], 0.140 to 0.871; p=0.024) and overall survival (HR, 0.322; 95% CI, 0.123 to 0.842; p=0.021). In patients with a PFI of less than 6 months, the difference of progression-free survival between patients with re-administration of cisplatin (3.0 months) and administration of cisplatin analogue (7.2 months) as second-line chemotherapy was statistically significant (p=0.049, log-rank test).
Conclusion: The concept of platinum sensitivity could be applied to recurrent cervical cancer and there is a possibility of noncross-resistance of cisplatin analogue with cisplatin.
Keywords: Cisplatin; Disease-Free Survival; Neoplasm Recurrence, Local; Platinum; Uterine Cervical Neoplasms.
Conflict of interest statement
Figures

Similar articles
-
Second platinum therapy in patients with uterine cervical cancer previously treated with platinum chemotherapy.Cancer Chemother Pharmacol. 2011 Aug;68(2):337-42. doi: 10.1007/s00280-010-1494-7. Epub 2010 Oct 26. Cancer Chemother Pharmacol. 2011. PMID: 20976599
-
Applicability of the concept of "platinum sensitivity" to recurrent endometrial cancer: the SGSG-012/GOTIC-004/Intergroup study.Gynecol Oncol. 2013 Dec;131(3):567-73. doi: 10.1016/j.ygyno.2013.09.021. Epub 2013 Sep 25. Gynecol Oncol. 2013. PMID: 24076450
-
Platinum-based combination chemotherapy vs. weekly cisplatin during adjuvant CCRT in early cervical cancer with pelvic LN metastasis.Anticancer Res. 2013 Oct;33(10):4675-81. Anticancer Res. 2013. PMID: 24123048
-
Medical therapy of advanced malignant epithelial tumours of the ovary.Forum (Genova). 2000 Oct-Dec;10(4):323-32. Forum (Genova). 2000. PMID: 11535983 Review.
-
Chemotherapy with paclitaxel, ifosfamide, and cisplatin for the treatment of squamous cell cervical cancer: the experience of Monza.Semin Oncol. 2000 Feb;27(1 Suppl 1):23-7. Semin Oncol. 2000. PMID: 10697040 Review.
Cited by
-
Role of NRF2 cascade in determining the differential response of cervical cancer cells to anticancer drugs: an in vitro study.Mol Biol Rep. 2022 Jan;49(1):109-119. doi: 10.1007/s11033-021-06848-2. Epub 2021 Oct 21. Mol Biol Rep. 2022. PMID: 34674139
-
Novel 1, 3-N, O-Spiroheterocyclic compounds inhibit heparanase activity and enhance nedaplatin-induced cytotoxicity in cervical cancer cells.Oncotarget. 2016 Jun 14;7(24):36154-36167. doi: 10.18632/oncotarget.8959. Oncotarget. 2016. PMID: 27166252 Free PMC article.
-
Laterally Extended Endopelvic Resection Versus Chemo or Targeted Therapy Alone for Pelvic Sidewall Recurrence of Cervical Cancer.Front Oncol. 2021 May 25;11:683441. doi: 10.3389/fonc.2021.683441. eCollection 2021. Front Oncol. 2021. PMID: 34113571 Free PMC article.
-
Phase II study of niraparib in recurrent or persistent rare fraction of gynecologic malignancies with homologous recombination deficiency (JGOG2052).J Gynecol Oncol. 2022 Jul;33(4):e55. doi: 10.3802/jgo.2022.33.e55. Epub 2022 May 3. J Gynecol Oncol. 2022. PMID: 35557035 Free PMC article. Review.
-
TNFAIP8 promotes cisplatin resistance in cervical carcinoma cells by inhibiting cellular apoptosis.Oncol Lett. 2019 May;17(5):4667-4674. doi: 10.3892/ol.2019.10076. Epub 2019 Feb 26. Oncol Lett. 2019. PMID: 30944654 Free PMC article.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Cadron I, Van Gorp T, Amant F, Leunen K, Neven P, Vergote I. Chemotherapy for recurrent cervical cancer. Gynecol Oncol. 2007;107(1 Suppl 1):S113–S118. - PubMed
-
- Thigpen T, Shingleton H, Homesley H, Lagasse L, Blessing J. Cisplatinum in treatment of advanced or recurrent squamous cell carcinoma of the cervix: a phase II study of the Gynecologic Oncology Group. Cancer. 1981;48:899–903. - PubMed
-
- Long HJ, 3rd, Bundy BN, Grendys EC, Jr, Benda JA, McMeekin DS, Sorosky J, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group Study. J Clin Oncol. 2005;23:4626–4633. - PubMed
-
- Omura GA, Blessing JA, Vaccarello L, Berman ML, Clarke-Pearson DL, Mutch DG, et al. Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1997;15:165–171. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

