Endothelial Connexin37 and Connexin40 participate in basal but not agonist-induced NO release
- PMID: 26198171
- PMCID: PMC4510910
- DOI: 10.1186/s12964-015-0110-1
Endothelial Connexin37 and Connexin40 participate in basal but not agonist-induced NO release
Abstract
Background: Connexin37 (Cx37) and Cx40 are crucial for endothelial cell-cell communication and homeostasis. Both connexins interact with endothelial nitric oxide synthase (eNOS). The exact contribution of these interactions to the regulation of vascular tone is unknown.
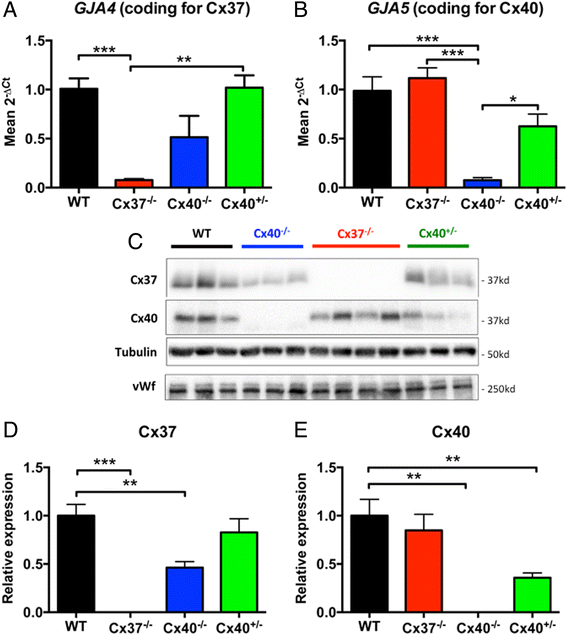
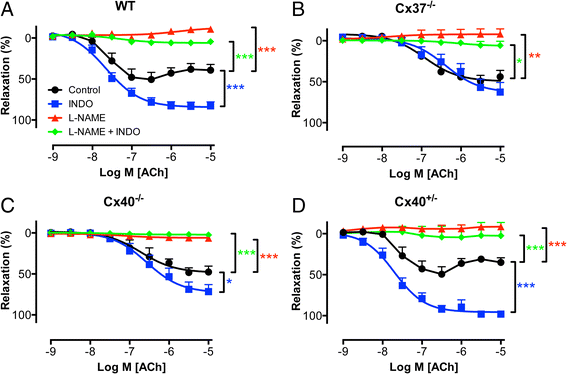
Results: Cx37 and Cx40 were expressed in close proximity to eNOS at cell-cell interfaces of mouse aortic endothelial cells. Absence of Cx37 did not affect expression of Cx40 and a 50 % reduction of Cx40 in Cx40(+/-) aortas did not affect the expression of Cx37. However, absence of Cx40 was associated with reduced expression of Cx37. Basal NO release and the sensitivity for ACh were decreased in Cx37(-/-) and Cx40(-/-) aortas but not in Cx40(+/-) aortas. Moreover, ACh-induced release of constricting cyclooxygenase products was present in WT, Cx40(-/-) and Cx40(+/-) aortas but not in Cx37(-/-) aortas. Finally, agonist-induced NO-dependent relaxations and the sensitivity for exogenous NO were not affected by genotype.
Conclusions: Cx37 is more markedly involved in basal NO release, release of cyclooxygenase products and the regulation of the sensitivity for ACh as compared to Cx40.
Figures



References
-
- Saez JC, Leybaert L. Hunting for connexin hemichannels. FEBS letters. 2014. doi:10.1016/j.febslet.2014.03.004. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

