Consensus statement for diagnosis of subcortical small vessel disease
- PMID: 26198175
- PMCID: PMC4758552
- DOI: 10.1038/jcbfm.2015.172
Consensus statement for diagnosis of subcortical small vessel disease
Abstract
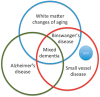
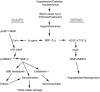
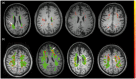
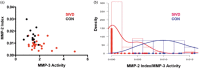
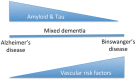
Vascular cognitive impairment (VCI) is the diagnostic term used to describe a heterogeneous group of sporadic and hereditary diseases of the large and small blood vessels. Subcortical small vessel disease (SVD) leads to lacunar infarcts and progressive damage to the white matter. Patients with progressive damage to the white matter, referred to as Binswanger's disease (BD), constitute a spectrum from pure vascular disease to a mixture with neurodegenerative changes. Binswanger's disease patients are a relatively homogeneous subgroup with hypoxic hypoperfusion, lacunar infarcts, and inflammation that act synergistically to disrupt the blood-brain barrier (BBB) and break down myelin. Identification of this subgroup can be facilitated by multimodal disease markers obtained from clinical, cerebrospinal fluid, neuropsychological, and imaging studies. This consensus statement identifies a potential set of biomarkers based on underlying pathologic changes that could facilitate diagnosis and aid patient selection for future collaborative treatment trials.
Figures

References
-
- Hachinski VC, Bowler JV. Vascular dementia. Neurology 1993; 43: 2159–2160. author reply 2160–2151. - PubMed
-
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study [see comments]. JAMA 1997; 277: 813–817. - PubMed
-
- Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer's disease [letter]. Lancet 1999; 354: 919–920. - PubMed
-
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007; 69: 2197–2204. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

