Modes of Human T Cell Leukemia Virus Type 1 Transmission, Replication and Persistence
- PMID: 26198240
- PMCID: PMC4517122
- DOI: 10.3390/v7072793
Modes of Human T Cell Leukemia Virus Type 1 Transmission, Replication and Persistence
Abstract
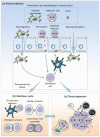
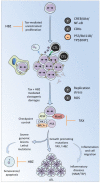
Human T-cell leukemia virus type 1 (HTLV-1) is a retrovirus that causes cancer (Adult T cell Leukemia, ATL) and a spectrum of inflammatory diseases (mainly HTLV-associated myelopathy-tropical spastic paraparesis, HAM/TSP). Since virions are particularly unstable, HTLV-1 transmission primarily occurs by transfer of a cell carrying an integrated provirus. After transcription, the viral genomic RNA undergoes reverse transcription and integration into the chromosomal DNA of a cell from the newly infected host. The virus then replicates by either one of two modes: (i) an infectious cycle by virus budding and infection of new targets and (ii) mitotic division of cells harboring an integrated provirus. HTLV-1 replication initiates a series of mechanisms in the host including antiviral immunity and checkpoint control of cell proliferation. HTLV-1 has elaborated strategies to counteract these defense mechanisms allowing continuous persistence in humans.
Keywords: HBZ; HTLV-1; Tax; viral persistence; viral replication.
Figures
References
-
- Bazarbachi A., Plumelle Y., Carlos Ramos J., Tortevoye P., Otrock Z., Taylor G., Gessain A., Harrington W., Panelatti G., Hermine O. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J. Clin. Oncol. 2010;28:4177–4183. doi: 10.1200/JCO.2010.28.0669. - DOI - PubMed
-
- Shiratori S., Yasumoto A., Tanaka J., Shigematsu A., Yamamoto S., Nishio M., Hashino S., Morita R., Takahata M., Onozawa M., et al. A retrospective analysis of allogeneic hematopoietic stem cell transplantation for adult T cell leukemia/lymphoma (ATL): Clinical impact of graft-versus-leukemia/lymphoma effect. Biol. Blood Marrow Transpl. 2008;14:817–823. doi: 10.1016/j.bbmt.2008.04.014. - DOI - PubMed
-
- Ishida T., Hishizawa M., Kato K., Tanosaki R., Fukuda T., Takatsuka Y., Eto T., Miyazaki Y., Hidaka M., Uike N., et al. Impact of graft-versus-host disease on allogeneic hematopoietic cell transplantation for adult T cell leukemia-lymphoma focusing on preconditioning regimens: Nationwide retrospective study. Biol. Blood Marrow Transpl. 2013;19:1731–1739. doi: 10.1016/j.bbmt.2013.09.014. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

