Daily oral iron supplementation during pregnancy
- PMID: 26198451
- PMCID: PMC8918165
- DOI: 10.1002/14651858.CD004736.pub5
Daily oral iron supplementation during pregnancy
Update in
-
Daily oral iron supplementation during pregnancy.Cochrane Database Syst Rev. 2024 Aug 15;8(8):CD004736. doi: 10.1002/14651858.CD004736.pub6. Cochrane Database Syst Rev. 2024. PMID: 39145520 Free PMC article.
Abstract
Background: Iron and folic acid supplementation has been the preferred intervention to improve iron stores and prevent anaemia among pregnant women, and it is thought to improve other maternal and birth outcomes.
Objectives: To assess the effects of daily oral iron supplements for pregnant women, either alone or in conjunction with folic acid, or with other vitamins and minerals as a public health intervention in antenatal care.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (10 January 2015). We also searched the WHO International Clinical Trials Registry Platform (ICTRP) (26 February 2015) and contacted relevant organisations for the identification of ongoing and unpublished studies (26 February 2015) .
Selection criteria: Randomised or quasi-randomised trials evaluating the effects of oral preventive supplementation with daily iron, iron + folic acid or iron + other vitamins and minerals during pregnancy.
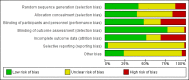
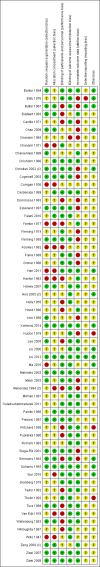
Data collection and analysis: We assessed the methodological quality of trials using standard Cochrane criteria. Two review authors independently assessed trial eligibility, extracted data and conducted checks for accuracy. We used the GRADE approach to assess the quality of the evidence for primary outcomes.We anticipated high heterogeneity among trials and we pooled trial results using a random-effects model and were cautious in our interpretation of the pooled results: the random-effects model gives the average treatment effect.
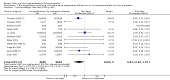
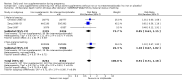
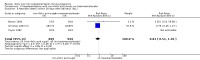
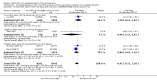
Main results: We included 61 trials. Forty-four trials, involving 43,274 women, contributed data and compared the effects of daily oral supplements containing iron versus no iron or placebo.Preventive iron supplementation reduced maternal anaemia at term by 70% (risk ratio (RR) 0.30; 95% confidence interval (CI) 0.19 to 0.46, 14 trials, 2199 women, low quality evidence), iron-deficiency anaemia at term (RR 0.33; 95% CI 0.16 to 0.69, six trials, 1088 women), and iron deficiency at term by 57% (RR 0.43; 95% CI 0.27 to 0.66, seven trials, 1256 women, low quality evidence). There were no clear differences between groups for severe anaemia in the second or third trimester, or maternal infection during pregnancy (RR 0.22; 95% CI 0.01 to 3.20, nine trials, 2125 women, very low quality evidence; and, RR 1.21; 95% CI 0.33 to 4.46; one trial, 727 women, low quality evidence, respectively), or maternal mortality (RR 0.33; 95% CI 0.01 to 8.19, two trials, 12,560 women, very low quality evidence), or reporting of side effects (RR 1.29; 95% CI 0.83 to 2.02, 11 trials, 2423 women, very low quality evidence). Women receiving iron were on average more likely to have higher haemoglobin (Hb) concentrations at term and in the postpartum period, but were at increased risk of Hb concentrations greater than 130 g/L during pregnancy, and at term.Compared with controls, women taking iron supplements less frequently had low birthweight newborns (8.4% versus 10.3%, average RR 0.84; 95% CI 0.69 to 1.03, 11 trials, 17,613 women, low quality evidence), and preterm babies (RR 0.93; 95% CI 0.84 to 1.03, 13 trials, 19,286 women, moderate quality evidence). They appeared to also deliver slightly heavier babies (mean difference (MD) 23.75; 95% CI -3.02 to 50.51, 15 trials, 18,590 women, moderate quality evidence). None of these results were statistically significant. There were no clear differences between groups for neonatal death (RR 0.91; 95% CI 0.71 to 1.18, four trials, 16,603 infants, low quality evidence), or congenital anomalies (RR 0.88, 95% CI 0.58 to 1.33, four trials, 14,636 infants, low quality evidence).Twenty-three studies were conducted in countries that in 2011 had some malaria risk in parts of the country. In some of these countries/territories, malaria is present only in certain areas or up to a particular altitude. Only two of these studies reported malaria outcomes. There is no evidence that iron supplementation increases placental malaria. For some outcomes heterogeneity was higher than 50%.
Authors' conclusions: Supplementation reduces the risk of maternal anaemia and iron deficiency in pregnancy but the positive effect on other maternal and infant outcomes is less clear. Implementation of iron supplementation recommendations may produce heterogeneous results depending on the populations' background risk for low birthweight and anaemia, as well as the level of adherence to the intervention.
Conflict of interest statement
We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review (e.g. employment, consultancy, stock ownership, honoraria, expert testimony).
Juan Pablo Peña‐Rosas was author of an excluded study on iron and folic acid intermittent supplementation.
Luz Maria De‐Regil is full‐time staff member of the Micronutrient Initiative, an International Organization that delivers vitamin interventions to children, women of reproductive age and pregnant women, including iron and folic acid supplementation in eight countries in Africa and South East Asia.
Disclaimer: Juan Pablo Peña‐Rosas is currently a staff member of the World Health Organization. Maria Nieves Garcia‐Casal is a Consultant working for the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the World Health Organization.
Therese Dowswell's institution (the University of Liverpool) has received an NIHR Cochrane Programme Grant and Therese is currently employed on this project. As part of her role, Therese helps volunteer review teams prepare Cochrane reviews. This review is not part of that portfolio of reviews. In the last 36 months Therese has received funding from the WHO to work on other Cochrane reviews. The Funders have no influence on the content or conclusions of the reviews Therese works on.
Figures













































































































































































































































































































Update of
-
Daily oral iron supplementation during pregnancy.Cochrane Database Syst Rev. 2012 Dec 12;12:CD004736. doi: 10.1002/14651858.CD004736.pub4. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2015 Jul 22;(7):CD004736. doi: 10.1002/14651858.CD004736.pub5. PMID: 23235616 Free PMC article. Updated.
References
References to studies included in this review
Barton 1994 {published data only (unpublished sought but not used)}
-
- Barton DPJ, Joy MT, Lappin TRJ, Afrasiabi M, Morel JG, O'Riordan J, et al. Maternal erythropoietin in singleton pregnancies: a randomized trial on the effect of oral hematinic supplementation. American Journal of Obstetrics and Gynecology 1994;170:896‐901. - PubMed
-
- Murphy JF. Randomised double blind control trial of iron/placebo administration to pregnant women with high booking Hb concentrations (Hb>149/dl). Personal communication 1988.
Batu 1976 {published data only}
-
- Batu AT, Toe T, Pe H, Nyunt KK. A prophylactic trial of iron and folic acid supplements in pregnant Burmese women. Israel Journal of Medical Sciences 1976;12:1410‐7. - PubMed
Butler 1967 {published and unpublished data}
-
- Butler EB. The effect of iron and folic acid in normal pregnancy (MD thesis). Vol. 1, Cardiff: Welsh National School of Medicine, 1967.
-
- Butler EB. The effect of iron and folic acid in normal pregnancy (MD thesis). Vol. 2 (Appendixes), Cardiff: Welsh National School of Medicine, Department of Obstetrics, 1967.
-
- Butler EB. The effect of iron and folic acid on red cell and plasma volume in pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1968;75:497‐510. - PubMed
Buytaert 1983 {published data only}
-
- Buytaert G, Wallenburg HCS, Eijk HG, Buytaert P. Iron supplementation during pregnancy. European Journal of Obstetrics & Gynecology and Reproductive Biology 1983;15:11‐6. - PubMed
Cantlie 1971 {published data only}
-
- Cantlie GSD, Leeuw NKM, Lowenstein L. Iron and folate nutrition in a group of private obstetrical patients. American Journal of Clinical Nutrition 1971;24:637‐41. - PubMed
Chan 2009 {published and unpublished data}
-
- Chan KK, Chan BC, Lam KF, Tam S, Lao TT. Iron supplement in pregnancy and development of gestational diabetes‐‐a randomised placebo‐controlled trial. BJOG: an international journal of obstetrics and gynaecology 2009;116(6):789‐98. - PubMed
Chanarin 1965 {published data only}
Chanarin 1971 {published data only}
Charoenlarp 1988 {published data only}
-
- Charoenlarp P, Dhanamitta S, Kaewvichit R, Silprasert A, Suwanaradd C, Na‐Nakorn S, et al. A WHO collaborative study on iron supplementation in Burma and in Thailand. American Journal of Clinical Nutrition 1988;47(2):280‐97. - PubMed
Chisholm 1966 {published data only}
-
- Chisholm M. A controlled clinical trial of prophylactic folic acid and iron in pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1966;73:191‐6. - PubMed
Christian 2003 (C) {published and unpublished data}
-
- Christian P. (personal communication) 02 October 2012.
-
- Christian P. (personal communication) 28 September 2007.
-
- Christian P, Darmstadt GL, Wu L, Khatry SK, LeClerq SC, Katz J, et al. The effect of maternal micronutrient supplementation on early neonatal morbidity in rural Nepal: a randomised, controlled, community trial. Archives of Disease in Childhood 2008;93(8):660‐4. - PubMed
-
- Christian P, Jiang T, Khatry SK, LeClerq SC, Shrestha SR, West KP Jr. Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal. American Journal of Clinical Nutrition 2006;83:788‐94. - PubMed
Cogswell 2003 {published and unpublished data}
-
- Cogswell ME, Parvanta I, Ickes L, Yip R, Brittenham GM. Individual patient data (as supplied 4 February 2004). Data on file.
-
- Cogswell ME, Parvanta I, Ickes L, Yip R, Brittenham GM. Iron supplementation during pregnancy, anemia, and birth weight: a randomized controlled trial. American Journal of Clinical Nutrition 2003;78(4):773‐81. - PubMed
-
- Cogswell ME, Parvanta I, Yip R, Brittenham GM. Iron supplementation during pregnancy for initially non‐anemic, iron replete women ‐ decreased prevalence of low birth weight infants. Report of the 2001 INACG Symposium. Why iron is important and what to do about it: a new perspective. Washington D.C.: ILSI Human Nutrition Institute, 2002; Vol. 1:42, Abstract no: 7.
-
- Cogswell ME, Parvanta I, Yip R, Brittenham GM. Low iron during pregnancy increases the risk of delivering preterm or small infants. Report of the 2001 INACG Symposium. Why iron is important and what to do about it: a new perspective. Washington DC: ILSI Human Nutrition Institute, 2002; Vol. 1:42, Abstract no: 8.
Corrigan 1936 {published data only}
-
- Corrigan JC, Strauss MB. The prevention of hypochromic anemia in pregnancy. JAMA 1936;106(13):1088‐90.
De Benaze 1989 {published data only}
-
- Benaze C, Galan P, Wainer R, Hercberg S. Prevention of iron deficient anemia during pregnancy by early iron supplementation: a controlled trial. Revue d Epidemiologie et de Sante Publique 1989;37:109‐18. - PubMed
Dommisse 1983 {published data only}
-
- Dommisse J, Bell DJH, Du Toit ED, Midgley V, Cohen M. Iron‐storage deficiency and iron supplementation in pregnancy. South African Medical Journal 1983;64:1047‐51. - PubMed
Eskeland 1997 {published and unpublished data}
-
- Eskeland B. Database provided by authors (as supplied 22 February 2004). Data on file.
-
- Eskeland B, Malterud K, Ulvik RJ, Hunskaar S. Iron supplementation in pregnancy: is less enough? A randomized, placebo controlled trial of low dose iron supplementation with and without heme iron. Acta Obstetricia et Gynecologica Scandinavica 1997;76(9):822‐8. - PubMed
Falahi 2010 {published data only}
-
- Falahi E. Effect of prophylactic iron supplementation on pregnancy outcome. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010) 2010.
-
- Falahi E, Akbari S, Ebrahimzade F, Gargari BP. Impact of prophylactic iron supplementation in healthy pregnant women on maternal iron status and birth outcome. Food and Nutrition Bulletin 2011;32(3):213‐7. - PubMed
Fenton 1977 {published data only}
-
- Fenton V, Cavill I, Fisher J. Iron stores in pregnancy. British Journal of Haematology 1977;37:145‐9. - PubMed
Fleming 1974 {published data only}
-
- Fleming AF, Martin JD, Hahnel R, Westlake AJ. Effects of iron and folic acid antenatal supplements on maternal haematology and fetal wellbeing. Medical Journal of Australia 1974;2:429‐36. - PubMed
Fleming 1985 {published data only}
-
- Fleming AF. Anaemia in pregnancy in the Guinea Savanna of Nigeria. In: Ludwig H, Thomsen K editor(s). Gynecology and Obstetrics. Berlin: Springer‐Verlag, 1986:122‐4.
-
- Fleming AF, Ghatoura GBS, Harrison KA, Briggs ND, Dunn DT. The prevention of anaemia in pregnancy in primigravidae in the guinea savanna of Nigeria. Annals of Tropical Medicine and Parasitology 1986;80:211‐33. - PubMed
-
- Harrison KA, Fleming AF, Briggs ND, Rossiter CE. Child‐bearing, health and social priorities: a survey of 22,774 consecutive hospital births in Zaria, Northern Nigeria. 5. Growth during pregnancy in Nigerian teenage primigravidae. British Journal of Obstetrics and Gynaecology 1985;92(5):32‐9. - PubMed
Foulkes 1982 {published data only}
-
- Foulkes J, Goldie DJ. The use of ferritin to assess the need for iron supplements in pregnancy. Journal of Obstetrics and Gynaecology 1982;3:11‐6.
Freire 1989 {published data only (unpublished sought but not used)}
-
- Freire WB. Hemoglobin as a predictor of response to iron therapy and its use in screening and prevalence estimates. American Journal of Clinical Nutrition 1989;50:1442‐9. - PubMed
Groner 1986 {published data only}
-
- Groner JA, Holtzman NA, Charney E, Mellits ED. A randomized trial of oral iron on tests of short‐term memory and attention span in young pregnant women. Journal of Adolescent Health Care 1986;7:44‐8. - PubMed
Han 2011 {published data only}
-
- Han XX, Sun YY, Ma AG, Yang F, Zhang FZ, Jiang DC, et al. Moderate NaFeEDTA and ferrous sulfate supplementation can improve both hematologic status and oxidative stress in anemic pregnant women. Asia Pacific Journal of Clinical Nutrition 2011;20(4):514‐20. - PubMed
Hankin 1963 {published data only}
-
- Hankin ME. The value of iron supplementation during pregnancy. Australian and New Zealand Journal of Obstetrics and Gynaecology 1963;3:111‐8. - PubMed
-
- Hankin ME, Symonds EM. Body weight, diet and pre‐eclamptic toxaemia in pregnancy. Australian and New Zealand Journal of Obstetrics and Gynaecology 1962;4:156‐60.
Harvey 2007 {published and unpublished data}
-
- Fairweather‐Tait S. Personal communication. 2007 September 5.
-
- Harvey LJ, Dainty JR, Hollands WJ, Bull VJ, Hoogewerff JA, Foxall RJ, et al. Effect of high‐dose iron supplements on fractional zinc absorption and status in pregnant women. American Journal of Clinical Nutrition 2007;85:131‐6. - PubMed
Hoa 2005 (C) {published data only}
-
- Hoa PT, Khan NC, Beusekom C, Gross R, Conde WL, Khoi HD. Milk fortified with iron or iron supplementation to improve nutritional status of pregnant women: an intervention trial from rural Vietnam. Food & Nutrition Bulletin 2005;26(1):32‐8. - PubMed
Holly 1955 {published data only}
-
- Holly RG. Anemia in pregnancy. Obstetrics & Gynecology 1955;5:562‐9. - PubMed
Hood 1960 {published data only}
-
- Hood WE, Bond WL. Iron deficiency prophylaxis during pregnancy. Obstetrics & Gynecology 1960;16:82‐4. - PubMed
Kerr 1958 {published data only}
-
- Kerr DNS, Davidson S. The prophylaxis of iron‐deficiency anemia in pregnancy. Lancet 1958;2:483‐8. - PubMed
Korkmaz 2014 {published data only}
-
- Korkmaz V, Ozkaya E, Seven BY, Duzguner S, Karsli MF, Kucukozkan T. Comparison of oxidative stress in pregnancies with and without first trimester iron supplement: a randomized double‐blind controlled trial. Journal of Maternal‐Fetal and Neonatal Medicine 2014;27(15):1535‐8. - PubMed
Kuizon 1979 {published data only}
-
- Kuizon MD, Platon TP, Ancheta LP, Angeles JC, Nunez CB, Macapinlac MP. Iron supplementation studies among pregnant women. Southeast Asian Journal of Tropical Medicine and Public Health 1979;10(4):520‐7. - PubMed
Lee 2005 {published and unpublished data}
-
- Lee JI, Lee JA, Lim HS. Effect of time of initiation and dose of prenatal iron and folic acid supplementation on iron and folate nutriture of Korean women during pregnancy. American Journal of Clinical Nutrition 2005;82(4):843‐9. - PubMed
-
- Lim HS. Personal communication 2007 September 27.
Liu 2000 {published data only}
-
- Liu M, He Y, Lu J. Study of the efficacy of ferroids tablets in preventing hypoferric anemia during pregnancy. New Chinese Medicine 2000;31(5):277‐8.
Liu 2012 {published data only}
-
- Cogswell ME. Impact of prenatal vitamin/mineral supplements on perinatal mortality. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006) 2006.
-
- Liu JM, Mei Z, Ye R, Serdula M, Li HT, Cogswell M. Impact of iron‐contained micronutrient supplementation on macrosomia and large for gestational age births. FASEB Journal 2012;26:1021‐2.
Ma 2010 {published data only}
-
- Ma AG, Schouten EG, Sun YY, Yang F, Han XX, Zhang FZ, et al. Supplementation of iron alone and combined with vitamins improves haematological status, erythrocyte membrane fluidity and oxidative stress in anaemic pregnant women. British Journal of Nutrition 2010;104(11):1655‐61. - PubMed
Makrides 2003 {published and unpublished data}
-
- Makrides M. Personal communication 2004 April 12.
-
- Makrides M, Crowther CA, Gibson RA, Gibson RS, Skeaff CM. Efficacy and tolerability of low‐dose iron supplements during pregnancy: a randomised controlled trial. American Journal of Clinical Nutrition 2003;78:145‐53. - PubMed
-
- Makrides M, Crowther CA, Gibson RA, Gibson RS, Skeaff CM. Low‐dose iron supplements in pregnancy prevent iron deficiency at the end of pregnancy and during the post‐partum period: the results of a randomised controlled trial [abstract]. Perinatal Society of Australia and New Zealand 7th Annual Congress; 2003 March 9‐12; Tasmania, Australia. 2003:P99.
-
- Parsons AG, Zhou SJ, Spurrier NJ, Makrides M. Effect of iron supplementation during pregnancy on the behaviour of children at early school age: long‐term follow‐up of a randomised controlled trial. British Journal of Nutrition 2008;99(5):1133‐9. - PubMed
-
- Zhou SH, Gibson RA, Crowther CA, Baghurst P, Makrides M. Effect of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 years of age: long term follow‐up of a randomized controlled trial. American Journal of Clinical Nutrition 2006;83(5):1112‐7. - PubMed
Meier 2003 {published data only}
Menendez 1994 (C) {published data only}
-
- Menendez C, Todd J, Alonso PL, Francis N, Lulat S, Ceesay S, et al. The effects or iron supplementation during pregnancy, given by traditional birth attendants, on the prevalence of anaemia and malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88:590‐3. - PubMed
-
- Menendez C, Todd J, Alonso PL, Francis N, Lulat S, Ceesay S, et al. The response to iron supplementation of pregnant women with the haemoglobin genotype AA or AS. Transactions of the Royal Society of Tropical Medicine and Hygiene 1995;89(3):289‐92. - PubMed
Milman 1991 {published data only}
-
- Milman N, Agger AO, Nielsen OJ. Iron supplementation during pregnancy. Effect on iron status markers, serum erythropoietin and human placental lactogen. A placebo controlled study in 207 Danish women. Danish Medical Bulletin 1991;38(6):471‐6. - PubMed
-
- Milman N, Agger AO, Nielson OJ. Iron status markers and serum erythropoietin in 120 mothers and newborn infants: effect of iron supplementation in normal pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1994;73:200‐4. - PubMed
-
- Milman N, Byg KE, Agger AO. Hemoglobin and erythrocyte indices during normal pregnancy and postpartum in 206 women with and without iron supplementation. Acta Obstetricia et Gynecologica Scandinavica 2000;79(2):89‐98. - PubMed
-
- Milman N, Graudal N, Agger AO. Iron status markers during pregnancy. No relationship between levels at the beginning of the second trimester, prior to delivery and post partum. Journal of Internal Medicine 1995;237:261‐7. - PubMed
-
- Milman N, Graudal N, Nielsen OJ, Agger AO. Serum erythropoietin during normal pregnancy: relationship to hemoglobin and iron status markers and impact of iron supplementation in a longitudinal, placebo‐controlled study on 118 women. International Journal of Hematology 1997;66(2):159‐68. - PubMed
Ouladsahebmadarek 2011 {published data only}
-
- Ouladsahebmadarek E, Sayyah‐Melli M, Taghavi S, Abbasalizadeh S, Seyedhejazie M. The effect of supplemental iron elimination on pregnancy outcome. Pakistan Journal of Medical Sciences 2011;27(3):641‐5.
Paintin 1966 {published and unpublished data}
-
- Paintin DB, Thompson AM, Hytten FE. Personal communication 1986.
-
- Paintin DB, Thomson AM, Hytten FE. Iron and haemoglobin level in pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1966;73:181‐90.
Preziosi 1997 {published data only}
-
- Preziosi P, Prual A, Galan P, Daouda H, Boureima H, Hercberg S. Effect of iron supplementation on the iron status of pregnant women: consequences for newborns. American Journal of Clinical Nutrition 1997;66:1178‐82. - PubMed
Pritchard 1958 {published data only}
-
- Pritchard J, Hunt C. A comparison of the hematologic responses following the routine prenatal administration of intramuscular and oral iron. Surgery, Gynecology and Obstetrics 1958;106:516‐8. - PubMed
Puolakka 1980 {published data only}
-
- Puolakka J, Janne O, Pakarinen A, Jarvinen PA, Vihko R. Serum ferritin as a measure of iron stores during and after normal pregnancy with and without iron supplement. Acta Obstetricia et Gynecologica Scandinavica 1980;95:43‐51. - PubMed
Romslo 1983 {published data only}
-
- Romslo I, Haram K, Sagen N, Augensen K. Iron requirements in normal pregnancy as assessed by serum ferritin, serum transferrin saturation and erythrocyte protoporphyrin determinations. British Journal of Obstetrics and Gynaecology 1983;90:101‐7. - PubMed
Siega‐Riz 2001 {published and unpublished data}
-
- Bodnar LM, Davidian M, Siega‐Riz AM, Tsiatis AA. Marginal structural models for analyzing causal effects of time‐dependent treatments: an application in perinatal epidemiology. American Journal of Epidemiology 2004;159(10):926‐34. - PubMed
-
- Jasti S, Siega‐Riz AM, Cogswell ME, Hartzema AG. Correction for errors in measuring adherence to prenatal multivitamin/mineral supplement use among low‐income women. Journal of Nutrition 2006;136(2):479‐83. - PubMed
-
- Jasti S, Siega‐Riz AM, Cogswell ME, Hartzema AG, Bentley ME. Pill count adherence to prenatal multivitamin/mineral supplement use among low‐income women. Journal of Nutrition 2005;135(5):1093‐101. - PubMed
-
- Siega‐Riz A, Hartzema A, Turnbull C, Thorp JJ, McDonald T. A trial of selective versus routine iron supplementation to prevent third trimester anemia during pregnancy. American Journal of Obstetrics and Gynecology 2001; Vol. 185, issue 6 Suppl:S119.
-
- Siega‐Riz AM, Hartzema AG, Turnbull C, Thorp J, McDonald T, Cogswell ME. The effects of prophylactic iron given in prenatal supplements on iron status and birth outcomes: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2006;194(2):512‐9. - PubMed
Simmons 1993 {published data only}
-
- Simmons WK, Cook JD, Bingham KC, Thomas M, Jackson J, Jackson M, et al. Evaluation of a gastric delivery system for iron supplementation in pregnancy. American Journal of Clinical Nutrition 1993;58:622‐6. - PubMed
Suharno 1993 {published data only}
-
- Suharno D, West CE, Karyadi D, Hautvast JGA. Supplementation with vitamin A and iron for nutritional anaemia in pregnant women in West Java, Indonesia. Lancet 1993;342:1325‐8. - PubMed
Sun 2010 {published data only}
-
- Sun YY, Ma AG, Yang F, Zhang FZ, Luo YB, Jiang DC, et al. A combination of iron and retinol supplementation benefits iron status, IL‐2 level and lymphocyte proliferation in anemic pregnant women. Asia Pacific Journal of Clinical Nutrition 2010;19(4):513‐9. - PubMed
Svanberg 1975 {published data only}
-
- Svanberg B, Arvidsson B, Norrby A, Rybo G, Solvell L. Absorption of supplemental iron during pregnancy ‐ a longitudinal study with repeated bone marrow studies and absorption measurements. Acta Obstetricia et Gynecologica Scandinavica 1975;48:87‐108. - PubMed
Taylor 1982 {published and unpublished data}
-
- Taylor DJ, Lind T. Red cell mass during and after normal delivery. British Journal of Obstetrics and Gynaecology 1979;86(5):364‐70. - PubMed
-
- Taylor DJ, Mallen C, McDougall N, Lind T. Personal communication 1982.
-
- Taylor DJ, Mallen C, McDougall N, Lind T. Effect of iron supplementation on serum ferritin levels during and after pregnancy. British Journal of Obstetrics and Gynaecology 1982;89:1011‐7. - PubMed
Tholin 1993 {published data only}
-
- Tholin K, Hallmans G, Sandstrom B, Goop M, Palm R, Abrahamsson M. Serum zinc, iron supplementation and pregnancy outcome. TEMA 8: Proceedings of the Eighth International Symposium on Trace Elements in Man and Animals; 1993 May 16‐22; Dresden, Germany. 1993:894‐5.
-
- Tholin K, Sandstrom B, Palm R, Hallmans G. Changes in blood manganese levels during pregnancy in iron supplemented and non supplemented women. Journal of Trace Elements in Medicine and Biology 1995;9(1):13‐7. - PubMed
Tura 1989 {published data only}
-
- Tura S, Carenza L, Baccarani M, Bagnara M, Bocci A, Bottone P, et al. Therapy and iron supplements with ferritin during pregnancy. A randomized prospective study of 458 cases. Recenti Progessi in Medicina 1989;80:607‐14. - PubMed
Van Eijk 1978 {published data only}
-
- Eijk HG, Kroos MJ, Hoogendoorn GA, Wallenburg HC. Serum ferritin and iron stores during pregnancy. Clinica Chimica Acta 1978;83(1‐2):81‐91. - PubMed
Wallenburg 1983 {published data only}
-
- Buytaert G, Wallenburg HCS, Eijk HG, Buytaert P. Iron supplementation during pregnancy. European Journal of Obstetrics & Gynecology and Reproductive Biology 1983;15:11‐6. - PubMed
-
- Wallenburg HCS, Eijk HG. Effect of oral iron supplementation during pregnancy on maternal and fetal iron status. Journal of Perinatal Medicine 1984;12:7‐12. - PubMed
Willoughby 1967 {published data only}
-
- Willoughby MLN. An investigation of folic acid requirements in pregnancy. II. British Journal of Haematology 1967;13:503‐9. - PubMed
Wills 1947 {published data only}
-
- Wills L, Hill G, Bingham K, Miall M, Wrigley J. Haemoglobin levels in pregnancy: the effect of the rationing scheme and routine administration of iron. British Journal of Nutrition 1947;1:126‐38. - PubMed
Zeng 2008 (C) {published data only (unpublished sought but not used)}
-
- Chang S, Zeng L, Brouwer ID, Kok FJ, Yan H. Effect of iron deficiency anemia in pregnancy on child mental development in rural China. Pediatrics 2013;131(3):e755‐e763. - PubMed
-
- Li Q, Yan H, Zeng L, Cheng Y, Liang W, Dang S, et al. Effects of maternal multimicronutrient supplementation on the mental development of infants in rural western China: follow‐up evaluation of a double‐blind, randomized, controlled trial. Pediatrics 2009;123(4):e685‐e692. - PubMed
-
- Wang W, Yan H, Zeng L, Cheng Y, Wang D, Li Q. No effect of maternal micronutrient supplementation on early childhood growth in rural western China: 30 month follow‐up evaluation of a double blind, cluster randomized controlled trial. European Journal of Clinical Nutrition 2012;66(2):261‐8. - PubMed
-
- Yan H. Impact of iron/folate versus multi‐micronutrient supplementation during pregnancy on birth weight: a randomised controlled trial in rural Western China. Current Controlled Trials (www.controlled‐trials.com) (accessed 15 February 2007) 2007.
Ziaei 2007 {published and unpublished data}
-
- Jafarbegloo E. Gastrointestinal complications of iron supplement in pregnant women. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010) 2010.
-
- Ziaei S. Personal communication 2007 October 1.
-
- Ziaei S, Norrozi M, Faghihzadeh S, Jafarbegloo E. A randomised placebo‐controlled trial to determine the effect of iron supplementation on pregnancy outcome in pregnant women with haemoglobin > or = 13.2 g/dl. BJOG: an international journal of obstetrics and gynaecology 2007;114(6):684‐8. - PubMed
Ziaei 2008 {published and unpublished data}
-
- Hamzehgardeshi Z. A randomized placebo‐controlled trial to determine the effect of iron supplementation on hematological indices in pregnant women with hemoglobin > 13.2 g/dl. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010) 2010.
-
- Janghorban R, Ziaei S, Faghihzade S. Evaluation of serum copper level in pregnant women with high hemoglobin. Iranian Journal of Medical Sciences 2006;31(3):170‐2.
-
- Ziaei S. Iron status markers in non‐anemic pregnant women with or without iron supplementation in pregnancy. Personal communication 2007 October 9.
-
- Ziaei S, Janghorban R, Shariatdoust S, Faghihzadeh S. The effects of iron supplementation on serum copper and zinc levels in pregnant women with high‐normal hemoglobin. International Journal of Gynecology & Obstetrics 2008;100:133‐5. - PubMed
-
- Ziaei S, Mehrnia M, Faghihzadeh S. Iron status markers in nonanemic pregnant women with and without iron supplementation. International Journal of Gynecology & Obstetrics 2008;100:130‐2. - PubMed
References to studies excluded from this review
Aaseth 2001 {published data only}
-
- Aaseth J, Thomassen Y, Ellingsen DG, Stoa‐Birketvedt G. Prophylactic iron supplementation in pregnant women in Norway. Journal of Trace Elements in Medicine & Biology 2001;15(2‐3):167‐74. - PubMed
Abel 2000 {published data only}
-
- Abel R, Rajaratnam J, Kalaimani A, Kirubakaran S. Can iron status be improved in each of the three trimesters? A community base study. European Journal of Clinical Nutrition 2000;54:490‐3. - PubMed
Adhikari 2009 {published data only}
-
- Adhikari K, Liabsuetrakul T, Pradhan N. Effect of education and pill count on hemoglobin status during prenatal care in Nepalese women: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2009;35(3):459‐66. - PubMed
Afifi 1978 {published data only}
-
- Afifi AM. Plexafer‐F in the management of latent iron deficiency in pregnancy. Journal of International Medical Research 1978;6:34‐40. - PubMed
Agrawal 2011 {published data only}
-
- Agrawal N. Impact of two oral iron supplementation regimens (intermittently and continuously/daily) for prevention of anaemia in pregnancy in women with normal haemoglobin levels. Clinical Trials Registry ‐ India (accessed 31 May 2013) 2011.
Ahn 2006 {published data only}
-
- Ahn E, Pairaudeau N, Pairaudeau N, Cerat Y, Couturier B, Fortier A, et al. A randomized cross over trial of tolerability and compliance of a micronutrient supplement with low iron separated from calcium vs high iron combined with calcium in pregnant women. BMC Pregnancy and Childbirth 2006;6:10. - PMC - PubMed
Alaoddolehei 2012 {published data only}
Angeles‐Agdeppa 2003 {published data only (unpublished sought but not used)}
-
- Angeles‐Agdeppa I. The effects of a community‐based weekly iron‐folate supplementation on hemoglobin and iron status of pregnant and non‐pregnant women in Philippines. Meeting on weekly iron/folic acid supplementation for preventing anaemia in women of reproductive age in the Western Pacific Region report; 2003 Sept 29 ‐ Oct 1; Manila, Philippines. Manila, Philippines: WHO, 2003.
-
- Angeles‐Agdeppa I, Paulino LS, Ramos AC, Etorma UM, Cavalli‐Sforza T, Milani S. Government‐industry partnership in weekly iron‐folic acid supplementation for women of reproductive age in the Philippines: impact on iron status. Nutrition Reviews 2005;63(12 Pt 2):S116‐S125. - PubMed
-
- Paulino LS, Angeles‐Agdeppa I, Etorma UM, Ramos AC, Cavalli‐Sforza T. Weekly iron‐folic acid supplementation to improve iron status and prevent pregnancy anemia in Filipino women of reproductive age: the Philippine experience through government and private partnership. Nutrition Reviews 2005;63(12 Pt 2):S109‐S115. - PubMed
Arija 2014 {published data only}
Babior 1985 {published data only}
-
- Babior BM, Peters WA, Briden PM, Cetrulo CL. Pregnant women's absorption of iron from prenatal supplements. Journal of Reproductive Medicine 1985;30:355‐7. - PubMed
Balmelli 1974 {published data only}
-
- Balmelli GP, Huser HJ. Folic acid deficiency in pregnant women in Switzerland [Zur Frage des Folsäuremangels beiSchwangeren in der Schweiz]. Schweizerische Medizinische Wochenschrift 1974;104(10):351‐6. - PubMed
Bencaiova 2007 {published data only (unpublished sought but not used)}
-
- Bencaiova G, Mandach U, Zimmerman R. Optimal prophylaxis of a lack of iron and iron‐deficiency anemia in the pregnancy: a randomized study [Optimale Prophylaxe e Ines Elsenmangels und elner Eisenmangelanamie In der Schwangerschaft: eine randomisierte Studie]. Gynakologisch‐Geburtshilfliche Rundschau 2007;47:140.
-
- Bencaiova G, Mandach U, Zimmermann R. Iron prophylaxis in pregnancy: intravenous route versus oral route. European Journal of Obstetrics & Gynecology and Reproductive Biology 2009;144(2):135‐9. - PubMed
Berger 2003 {published data only (unpublished sought but not used)}
-
- Berger J. Effectiveness of weekly iron/folate supplementation on anaemia and iron status in women of reproductive age in rural Viet Nam. Meeting on weekly iron/folic acid supplementation for preventing anaemia in women of reproductive age in the Western Pacific Region report; 2003 Sept 29‐Oct 1; Manila, Philippines. Manila, Philippines: WHO, 2003.
-
- Berger J, Thanh HT, Cavalli‐Sforza T, Smitasiri S, Khan NC, Milani S, et al. Community mobilization and social marketing to promote weekly iron‐folic acid supplementation in women of reproductive age in Vietnam: impact on anemia and iron status. Nutrition Reviews 2005;63(12 Pt 2):S95‐S108. - PubMed
-
- Hoa PT, Berger J, Paliakara N, Nhien NV, Morestin‐Cadet S, Quyen DT, et al. Weekly iron‐folate supplementation in women in reproductive age in Vietnam: a new approach to control iron deficiency anemia during pregnancy. INACG Symposium; 2001 Feb 15‐16; Hanoi, Vietnam. 2001:45, Abstract No: 18.
-
- Khan NC, Thanh HT, Berger J, Hoa PT, Quang ND, Smitasiri S, et al. Community mobilization and social marketing to promote weekly iron‐folic acid supplementation: a new approach toward controlling anemia among women of reproductive age in Vietnam. Nutrition Reviews 2005;63(12 Pt 2):S87‐S94. - PubMed
Bergsjo 1987 {published data only}
-
- Bergsjo P. The effects of iron supplementation in pregnancy. Personal communication 1987.
Bhatla 2009 {published data only}
-
- Bhatla N, Kaul N, Lal N, Kriplani A, Agarwal N, Saxena R, et al. Comparison of effect of daily versus weekly iron supplementation during pregnancy on lipid peroxidation. Journal of Obstetrics and Gynaecology Research 2009;35(3):438‐45. - PubMed
Blot 1980 {published data only}
-
- Blot I, Papiernik E, Kalwatsser JP, Werner E, Tchernia G. Influence of routine administration of folic acid and iron during pregnancy. Gynecologic and Obstetric Investigation 1981;12:294‐304. - PubMed
-
- Blot I, Tchernia G, Chenayer M, Hill C, Hajeri H, Leluc R. Iron deficiency in the pregnant woman. Its repercussions on the newborn. The influence of systematic iron treatment. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1980;9:489‐95. - PubMed
-
- Tchernia G, Blot I, Rey A, Kaltwasser JP, Zittoun J, Papiernik E. Maternal folate status, birthweight and gestational age. Developmental Pharmacology Therapeutics 1982;4 Suppl:58‐65. - PubMed
-
- Zittoun J, Blot I, Hill C, Zittoun R, Papiernik E, Tchernia G. Iron supplements vs placebo during pregnancy: its effects on iron and folate status on mothers and newborns. Annals of Nutrition and Metabolism 1983;27:320‐7. - PubMed
Bokhari 2011 {published data only}
-
- Bokhari EJ. A functional food for the prevention of iron‐deficiency anemia. ClinicalTrial.gov (http://clinicaltrials.gov) (accessed 19 July 2011) 2011.
-
- Bokhari F, Derbyshire EJ, Li W, Brennan CS. Can an iron‐rich staple food help women to achieve dietary targets in pregnancy?. International Journal of Food Sciences & Nutrition 2012;63(2):199‐207. - PubMed
Bokhari 2012 {published data only}
-
- Bokhari F, Derbyshire EJ, Hickling D, Li W, Brennan CS. A randomized trial investigating an iron‐rich bread as a prophylaxis against iron deficiency in pregnancy. International Journal of Food Sciences & Nutrition 2012;63(4):461‐7. - PubMed
Brown 1972 {published data only}
-
- Brown GM, Dawson DW. Prevention of anaemia in pregnancy. Current Medical Research and Opinion 1972;1:93‐9. - PubMed
Burslem 1968 {published data only}
-
- Burslem RW, Poller L, Wacks H. A trial of slow release ferrous sulphate (Ferrogradumet) in prevention of iron deficiency in pregnancy. Acta Haematologica 1968;40:200‐4. - PubMed
Buss 1981 {published data only}
-
- Buss M. Therapy of iron‐folic acid deficiency in pregnancy [Therapie des Eisen‐Folsauremangels in der Schwangerschaft]. Zeitschrift fur Allgemeinmedizin 1981;57(22):1526‐32. - PubMed
Carrasco 1962 {published data only}
-
- Carrasco E, Jose F, Samson G, Germar E, Padilla B. Effect of D‐sorbitol on the absorption and transfer of nutrients from mother to fetus. American Journal of Clinical Nutrition 1962;11:533‐6. - PubMed
Casanueva 2003a {published and unpublished data}
-
- Casanueva E. Weekly iron‐folate (Fe‐fol) supplementation during pregnancy in Mexican women. Personal communication 2003.
-
- Casanueva E, Viteri FE, Mares‐Galindo M, Meza‐Camacho C, Loria A, Schnaas L, et al. Weekly iron as a safe alternative to daily supplementation for nonanemic pregnant women. Archives of Medical Research 2006;37(5):674‐82. - PubMed
Castren 1968 {published data only}
-
- Castren O, Levanto A, Rauramo L, Ruponen S. Preventive iron and folic acid therapy in pregnancy. Annales Chirurgiae et Gynaecologiae Fenniae 1968;57:382‐6. - PubMed
Chanarin 1968 {published data only}
Chawla 1995 {published data only}
-
- Chawla PK, Puri R. Impact of nutritional supplements on hematological profile of pregnant women. Indian Pediatrics 1995;32:876‐80. - PubMed
Chew 1996a {published and unpublished data}
-
- Chew F, Torun B, Viteri FE. Comparison of weekly and daily iron supplementation to pregnant women in Guatemala (supervised and unsupervised). FASEB Journal 1996;10:A4221.
-
- Chew F, Torun B, Viteri FE. Individual patient data (as supplied 15 January 2004). Data on file.
-
- Chew F, Torún B, Viteri FE. Comparison of daily and weekly iron supplementation in pregnant women with and without direct supervision [Comparación de la suplementación diaria o semanal de hierro en mujeres embarazadas con y sin supervisión directa]. XI Congreso Latino Americano de Nutrición, Libro de Resumenes. Guatemala: SLAN, 1997:94.
Chew 1996b {published and unpublished data}
-
- Chew F, Torun B, Viteri FE. Comparison of weekly and daily iron supplementation to pregnant women in Guatemala (supervised and unsupervised). FASEB Journal 1996;10:A4221.
-
- Chew F, Torun B, Viteri FE. Individual patient data (as supplied 15 January 2004). Data on file.
-
- Chew F, Torún B, Viteri FE. Comparison of daily and weekly iron supplementation in pregnant women with and without direct supervision [Comparación de la suplementación diaria o semanal de hierro en mujeres embarazadas con y sin supervisión directa]. XI Congreso Latino Americano de Nutrición, Libro de Resumenes. Guatemala: SLAN, 1997:94.
Coelho 2000 {published data only}
-
- Coelho K, Ramdas S, Pillai S. A comparative study of changes in haemoglobin with high and low dose iron preparations in pregnant women. Journal of Obstetrics and Gynecology of India 2000;50(2):37‐9.
Cook 1990 {published data only}
-
- Cook JD, Carriaga M, Kahn SG, Schalch W, Skikne BS. Gastric delivery system for iron supplementation. Lancet 1990;335(8698):1136‐9. - PubMed
Dawson 1962 {published data only}
-
- Dawson DW, More JRS, Aird DC. Prevention of megaloblastic anaemia in pregnancy by folic acid. Lancet 1962;2:1015‐20. - PubMed
Dawson 1987 {published data only}
-
- Dawson EB, McGanity WJ. Protection of maternal iron stores in pregnancy. Journal of Reproductive Medicine 1987;32(6 Suppl):478‐87. - PubMed
Dijkhuizen 2004 {published data only}
-
- Dijkhuizen MA, Wieringa FT, West CE, Muhilal. Zinc plus beta‐carotene supplementation of pregnant women is superior to beta‐carotene supplementation alone in improving vitamin A status in both mothers and infants. American Journal of Clinical Nutrition 2004;80(5):1299‐307. - PubMed
Edgar 1956 {published data only}
-
- Edgar W, Rice HM. Administration of iron in antenatal clinics. Lancet 1956;1:599‐602. - PubMed
Ekstrom 1996 {published data only}
-
- Ekstrom EM, Kavishe FP, Habicht J, Frongillo EA, Rasmussen KM, Hemed L. Adherence to iron supplementation during pregnancy in Tanzania: determinants and hematologic consequences. American Journal of Clinical Nutrition 1996;64:368‐74. - PubMed
Ekstrom 2002 {published and unpublished data}
-
- Ekstrom EC. Personal communication 2004 April 12.
-
- Ekstrom EC, Hyder SM, Chowdhury AM, Chowdhury SA, Lonnerdal B, Habicht JP, et al. Efficacy and trial effectiveness of weekly and daily iron supplementation among pregnant women in rural Bangladesh: disentangling the issues. American Journal of Clinical Nutrition 2002;76(6):1392‐400. - PubMed
-
- Hyder SM, Persson LA, Chowdhury AM, Ekstrom EC. Do side‐effects reduce compliance to iron supplementation? A study of daily‐ and weekly‐dose regimens in pregnancy. Journal of Health, Population and Nutrition 2002;2:175‐9. - PubMed
-
- Hyder SM, Persson LA, Chowdhury R, Lonnerdal B, Ekstrom EC. Impact of daily and weekly iron supplementation to women in pregnancy and puerperium on haemoglobin and iron status six weeks postpartum: results from a community‐based study in Bangladesh. Scandinavian Journal of Nutrition 2003;47(1):19‐25.
Fletcher 1971 {published data only}
-
- Fletcher J, Gurr A, Fellingham F, Prankerd T, Brant H, Menzies D. The value of folic acid supplements in pregnancy. Journal of Obstetrics and Gynaecology of the British Empire 1971;78:781‐5. - PubMed
Giles 1971 {published data only}
-
- Giles PF, Harcourt AG, Whiteside MG. The effect of prescribing folic acid during pregnancy on birthweight and duration of pregnancy. A double blind trial. Medical Journal of Australia 1971;2:17‐21. - PubMed
Gomber 2002 {published data only}
-
- Gomber S, Agarwal KN, Mahajan C, Agarwal N. Impact of daily versus weekly hematinic supplementation on anemia in pregnant women. Indian Pediatrics 2002;39(4):339‐46. - PubMed
Goonewardene 2001 {published data only}
-
- Goonewardene M, Liyanage C, Fernando R. Intermittent oral iron supplementation during pregnancy. Ceylon Medical Journal 2001;46(4):132‐5. - PubMed
Gopalan 2004 {published data only}
-
- Gopalan S, Patnaik R, Ganesh K. Feasible strategies to combat low birth weight and intra‐uterine growth retardation. Journal of Pediatric Gastroenterology and Nutrition 2004;39(Suppl 1):S37.
Goshtasebi 2012 {published data only}
-
- Goshtasebi A, Alizadeh M. Impact of twice weekly versus daily iron supplementation during pregnancy on maternal and fetal haematological indices: A randomized clinical trial [lImpact d'une prise de complement en fer bihebdomadaire par rapport a une prise quotidienne pendant la grossesse sur des indices hematologiques maternels et foetaux: Une etude clinique randomisee]. Eastern Mediterranean Health Journal 2012;18(6):561‐6. - PubMed
Gringras 1982 {published data only}
-
- Gringras M. A comparison of two combined iron‐folic acid preparations in the prevention of anaemia in pregnancy. Journal of International Medical Research 1982;10:268‐70. - PubMed
Grover 1998 {published data only}
-
- Grover V, Aggarwal OP, Gupta A, Kumar P, Tiwari RS. Effect of daily and alternate day iron and folic acid supplementation to pregnant females on the weight of the newborn. Indian Journal of Community Medicine 1998;23(4):165‐8.
Guldholt 1991 {published data only}
-
- Guldholt IS, Trolle BG, Hvidman LE. Iron supplementation during pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1991;70:9‐12. - PubMed
Hampel 1974 {published data only}
-
- Hampel K, Roetz R. Influence of a long‐time substitution with a folate‐iron combination in pregnancy on serum folate and serum iron and on hematological parameters. Geburtshilfe und Frauenheilkunde 1974;34:409‐17. - PubMed
Hanieh 2013 {unpublished data only}
-
- Biggs BA. A randomised controlled trial to compare the impact on birth weight of daily iron‐folic acid, twice weekly iron‐folic acid and twice weekly multiple micronutrient supplementation for pregnant women in Ha Nam province, Vietnam. Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) (accessed 17 January 2011) 2011.
Hartman‐Craven 2009 {published data only}
Hawkins 1987 {published data only}
-
- Hawkins DF. Relative efficacy of sustained release iron and iron with folic acid treatment in pregnancy. Personal communication 1987.
Hemminki 1991 {published and unpublished data}
-
- Hemminki E, Merilainen J. Long‐term effects of iron prophylaxis during pregnancy. International Journal of Gynecology & Obstetrics 1994;46:3.
-
- Hemminki E, Merilainen J. Long‐term follow‐up of mothers and their infants in a randomized trial on iron prophylaxis during pregnancy. American Journal of Obstetrics and Gynecology 1995;173:205‐9. - PubMed
-
- Hemminki E, Rimpela U. A randomized comparison of routine vs selective iron supplementation during pregnancy. Journal of the American College of Nutrition 1991;10:3‐10. - PubMed
-
- Hemminki E, Rimpela U, Yla‐Outinen A. Iron prophylaxis during pregnancy and infections. International Journal of Vitamin and Nutrition Research 1991;61:370‐1. - PubMed
Hermsdorf 1986 {published data only}
-
- Hermsdorf J, Ring D, Retzke U, Bruschke G. Oral iron prophylaxis during pregnancy. A longitudinal study about hematologic and clinical parameters in treated and non‐treated pregnant women. Proceedings of 10th European Congress of Perinatal Medicine; 1986 August 12‐16; Leipzig, Germany. 1986:84.
Horgan 1966 {published data only}
-
- Horgan M, Woodliff M, Mangion J. A combined iron and folic‐acid preparation in the prophylaxis of anaemia of pregnancy. Practitioner 1966;197:683‐6. - PubMed
Hosokawa 1989 {published data only}
-
- Hosokawa K. Studies on anemia in pregnant women: therapeutic efficacy of iron monotherapy vs. combination therapy with iron and vitamin C. Rinsho to Kenkyu (The Japanese Journal of Clinical and Experimental Medicine) 1989;66(10):3329‐35.
Hossain 2014 {published data only}
-
- Hossain N, Kanani FH, Ramzan S, Kausar R, Ayaz S, Khanani R, et al. Obstetric and neonatal outcomes of maternal vitamin D supplementation: Results of an open label randomized controlled trial of antenatal vitamin D supplementation in Pakistani women. Journal of Clinical Endocrinology and Metabolism 2014;99(7):2448‐55. - PubMed
Itam 2003 {published data only}
-
- Itam IH. The effect of "Chemiron" on haematological parameters and ferritin levels in pregnant Nigerian women in Calabar. Mary Slessor Journal of Medicine 2003;3(2):17‐24.
Iyengar 1970 {published data only}
-
- Iyengar L. Effect of folic acid supplementation on birth weight of infants. American Journal of Obstetrics and Gynaecology 1974;122:332‐6. - PubMed
-
- Iyengar L. Folic acid requirements of Indian pregnant women. American Journal of Obstetrics and Gynecology 1971;111(1):13‐6. - PubMed
-
- Iyengar L, Apte SV. Prophylaxis of anemia in pregnancy. American Journal of Clinical Nutrition 1970;23:725‐30. - PubMed
Kaestel 2005 {published data only}
-
- Andersen GS, Friis H, Michaelsen KF, Rodrigues A, Benn CS, Aaby P, et al. Effects of maternal micronutrient supplementation on fetal loss and under‐2‐years child mortality: long‐term follow‐up of a randomised controlled trial from Guinea‐Bissau. African Journal of Reproductive Health 2010;14(2):17‐26. - PubMed
-
- Kaestel P, Michaelsen KF, Aaby P, Friis H. Effects of prenatal multimicronutrient supplements on birth weight and perinatal mortality: a randomised, controlled trial in Guinea‐Bissau. European Journal of Clinical Nutrition 2005;59(9):1081‐9. - PubMed
Kann 1988 {published data only}
-
- Kann J, Lyon JA, Bon C. Availability of iron from four prenatal multivitamin/multimineral products. Clinical Therapeutics 1988;10:287‐93. - PubMed
Khambalia 2009 {published data only}
-
- Khambalia A. Periconceptional iron supplementation and iron and folate status among pregnant and non‐pregnant women in rural Bangladesh [thesis]. Toronto: University of Toronto, 2009.
-
- Khambalia A, O'Connor D, Zlotkin S. Reduced anemia and improved iron and folate status before and after pregnancy among females in rural Bangladesh is related to length of adherence to periconceptional iron and folic acid supplementation. Faseb Journal 2014;23(1):Abstract no: 917.6.
-
- Khambalia AZ, O'Connor DL, Macarthur C, Dupuis A, Zlotkin SH. Periconceptional iron supplementation does not reduce anemia or improve iron status among pregnant women in rural Bangladesh. American Journal of Clinical Nutrition 2009;90(5):1295‐302. - PubMed
Kulkarni 2010 {published data only}
-
- Kulkarni B, Christian P, LeClerq SC, Khatry SK. Determinants of compliance to antenatal micronutrient supplementation and women's perceptions of supplement use in rural Nepal. Public Health Nutrition 2010;13(1):82‐90. - PubMed
Kumar 2005 {published data only}
-
- Kumar A, Jain S, Singh NP, Singh T. Oral versus high dose parenteral iron supplementation in pregnancy. International Journal of Gynecology & Obstetrics 2005;89:7‐13. - PubMed
Lira 1989 {published data only}
-
- Lira P, Barrena N, Foradori A, Gormaz G, Grebe G. Folate deficiency in pregnancy: effect of supplementary folic acid [Deficienca de folatos en el embarazo: efecto de una suplementacion con acido folico]. Sangre 1989;34(1):24‐7. - PubMed
Liu 1996 {published and unpublished data}
-
- Liu XN, Liu PY. The effectiveness of weekly iron supplementation regimen in improving the iron status of Chinese children and pregnant women. Biomedical and Environmental Sciences 1996;9:341‐7. - PubMed
-
- Liu XN, Liu PY, Viteri FE. Individual patient data (as supplied December 2003). Data on file.
Ma 2008 {published data only}
-
- Ma AG, Schouten EG, Zhang FZ, Kok FJ, Yang F, Jiang DC, et al. Retinol and riboflavin supplementation decreases the prevalence of anemia in Chinese pregnant women taking iron and folic acid supplements. Journal of Nutrition 2008;138(10):1946‐50. - PubMed
Madan 1999 {published data only}
-
- Madan N, Prasannaraj P, Rusia U, Sundaram KR, Nath LM, Sood SK. Monitoring oral iron therapy with protoporphyrin/heme ratios in pregnant women. Annals of Hematology 1999;78(6):279‐83. - PubMed
Marin 2012 {published data only}
Mbaye 2006 {published data only}
-
- Mbaye A, Richardson K, Balajo B, Dunyo S, Shulman C, Milligan P, et al. Lack of inhibition of the anti‐malarial action of sulfadoxine‐pyrimethamine by folic acid supplementation when used for intermittent preventive treatment in Gambian primigravidae. American Journal of Tropical Medicine & Hygiene 2006;74(6):960‐4. - PubMed
McKenna 2002 {published data only (unpublished sought but not used)}
-
- McKenna D, Spence D, Dornan J. A randomised, double‐blind, placebo‐controlled trial investigating the place of spatone‐iron plus as a prophylaxis against iron deficiency in pregnancy [abstract]. Journal of Obstetrics and Gynaecology 2002;22(2 Suppl):S45.
-
- McKenna D, Spence D, Haggan SE, McCrum E, Dornan JC, Lappin TR. A randomised trial investigating an iron‐rich natural mineral water as a prophylaxis against iron deficiency in pregnancy. Clinical and Laboratory Haematology 2003;25(2):99‐103. - PubMed
Menon 1962 {published data only}
-
- Menon MKK, Rajan L. Prophylaxis of anaemia in pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1962;12:382‐9.
Metz 1965 {published data only}
-
- Metz J, Festenstein H, Welch P. Effect of folic acid and vitamin B12 supplementation on test of folate and Vitamin B12 nutrition in pregnancy. American Journal of Clinical Nutrition 1965;16:472‐9. - PubMed
Milman 2005 {published data only}
-
- Milman N, Bergholt T. Postpartum anemia ‐ Defining threshold hemoglobin values. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S829.
-
- Milman N, Bergholt T, Eriksen L, Byg KE, Graudal N, Pedersen P, et al. Iron prophylaxis during pregnancy ‐ how much iron is needed? A randomized dose‐response study of 20‐80 mg ferrous iron daily in pregnant women. Acta Obstetricia et Gynecologica Scandinavica 2005;84:238‐47. - PubMed
-
- Milman N, Byg KE, Bergholt T, Eriksen L. Side effects of oral iron prophylaxis in pregnancy ‐ myth or reality?. Acta Haematologica 2006;115(1‐2):53‐7. - PubMed
-
- Milman N, Byg KE, Bergholt T, Eriksen L, Hvas AM. Body iron and individual iron prophylaxis in pregnancy ‐ should the iron dose be adjusted according to serum ferritin?. Annals of Hematology 2006;85(9):567‐73. - PubMed
Milman 2014 {published data only}
-
- Milman N, Jonsson L, Dyre P, Pedersen PL, Larsen LG. Ferrous bisglycinate 25 mg iron is as effective as ferrous sulfate 50 mg iron in the prophylaxis of iron deficiency and anemia during pregnancy in a randomized trial. Journal of Perinatal Medicine 2014;42(2):197‐206. - PubMed
Mitra 2012 {published data only}
-
- Mitra AK, Khoury AJ. Universal iron supplementation: a simple and effective strategy to reduce anaemia among low‐income, postpartum women. Public Health Nutrition 2012;15(3):546‐53. - PubMed
Morgan 1961 {published data only}
-
- Morgan EH. Plasma‐iron and haemoglobin levels in pregnancy. Lancet 1961;1:9‐12. - PubMed
-
- Morgan EH. Plasma‐iron and haemoglobin levels in pregnancy. Personal communication 1987 January 19.
Morrison 1977 {published data only}
-
- Morrison J, Bell J, Chang AMZ, Larkin PK. A comparative trial of haematinic supplements in pregnancy. Medical Journal of Australia 1977;1:482‐4. - PubMed
Mukhopadhyay 2004 {published data only}
-
- Mukhopadhyay A, Bhatla N, Kriplani A, Agarwal N, Saxena R. Erythrocyte indices in pregnancy: effect of intermittent iron supplementation. National Medical Journal of India 2004;17(3):135‐7. - PubMed
-
- Mukhopadhyay A, Bhatla N, Kriplani A, Pandey RM, Saxena R. Daily versus intermittent iron supplementation in pregnant women: hematological and pregnancy outcome. Journal of Obstetrics and Gynaecology Research 2004;30(6):409‐17. - PubMed
Mumtaz 2000 {published data only}
-
- Mumtaz Z, Shahab S, Butt N, Rab MA, DeMuynck A. Daily iron supplementation is more effective than twice weekly iron supplementation in pregnant women in Pakistan in a randomized double‐blind clinical trial. Journal of Nutrition 2000;130(11):2697‐702. - PubMed
Nguyen 2008 {published data only}
-
- Gill SK, Nguyen P, Koren G. Adherence and tolerability of iron‐containing prenatal multivitamins in pregnant women with pre‐existing gastrointestinal conditions. Journal of Obstetrics and Gynaecology 2009;29(7):594‐8. - PubMed
Nogueira 2002 {published data only}
-
- Nogueira NDN, Macedo ADS, Parente JV, Cozzolino SMF. Nutritional profile of newborns of adolescent mothers supplemented with iron, in different concentrations, zinc and pholic acid. Revista de Nutricao 2002;15:193‐200.
-
- Nogueira Ndo N, Parente JV, Cozzolino SM. Changes in plasma zinc and folic acid concentrations in pregnant adolescents submitted to different supplementation regimens. Cadernos de Saude Publica 2003;19(1):155‐60. - PubMed
Ogunbode 1984 {published data only}
-
- Ogunbode O, Damole IO. Prophylaxis of anaemia in obstetric patients: administration of Ferrograd Folic 500 Plus compared with conventional iron and folic supplementation. Current Therapeutic Research, Clinical and Experimental 1984;35:1043‐8.
Ogunbode 1992 {published data only}
-
- Ogunbode O, Otubu JAM, Akeredolu OO, Akintunde EA, Olatunji PO, Jolayemi ET. The effect of Chemiron capsules on maternal and fetal hematologic indices, including birth weight. Current Therapeutic Research, Clinical and Experimental 1992;51:634‐46.
-
- Ogunbode O, Otubu JAM, Briggs ND, Adeleye JA. Chemiron ‐ A new hematinic preparation. How effective during pregnancy?. Current Therapeutic Research, Clinical & Experimental 1992;51:163‐73.
Ortega‐Soler 1998 {unpublished data only}
-
- Ortega‐Soler CR, Langini SH, Fleishman S, Lopez LB, Garcia M, Guntin R, et al. Iron nutritional status in pregnant women with and without iron supplementation [Estado nutricional con respecto al hierro (Fe) en gestantes con y sin suplementacion]. Personal communication 1998.
Osifo 1970 {published data only}
-
- Osifo BO. The effect of folic acid and iron in the prevention of nutritional anaemias in pregnancy in Nigeria. British Journal of Nutrition 1970;24(3):689‐94. - PubMed
Osrin 2005 {published data only}
-
- Adhikari R, Manandhar D, Costello A, Tompkins A, Filteau S, Osrin D, et al. The effects of antenatal multiple micronutrient supplementation on birthweight, gestation and infection: a double blind, randomised controlled trial conducted in Nepal: study protocol. MIRA Janakpur Multiple Micronutrient Supplementation 2003.
-
- Osrin D, Vaidya A, Shrestha Y, Baniya RB, Manandhar DS, Adhikari RK, et al. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double‐blind, randomised controlled trial. Lancet 2005;365:955‐62. - PubMed
Parkkali 2013 {published data only}
-
- Hemminki E. Routine iron prophylaxis during pregnancy (PROFEG). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
-
- Parkkali S, Abacassamo F, Nwaru BI, Salome G, Augusto O, Regushevskaya E, et al. Comparison of routine prenatal iron prophylaxis and screening and treatment for anaemia: Pregnancy results and preliminary birth results from a pragmatic randomised controlled trial (PROFEG) in Maputo, Mozambique. BMJ Open 2013;3(2):001948. - PMC - PubMed
-
- Parkkali S, Abacassamo F, Salome G, Augusto O, Nikula M, on behalf of the PROFEG‐group. Routine iron prophylaxis during pregnancy. Effects on maternal and child health in Maputo City and the urban part of Maputo Province, Mozambique. Report of a pilot study. Profeg Group, 2007.
Payne 1968 {published data only}
Peña‐Rosas 2003 {published data only}
-
- Pena‐Rosas JP, Nesheim M, Garcia‐Casal MN, Crompton DWT, Sanjur D, Viteri FE, et al. Intermittent iron supplementation regimens are able to maintain safe maternal hemoglobin concentrations during pregnancy in Venezuela. Journal of Nutrition 2004;134(5):1099‐104. - PubMed
Picha 1975 {published data only}
-
- Picha E. Iron treatment by effervescent tablets [Ein neuer Weg der Eisentherapie]. Geburtshilfe und Frauenheilkunde 1975;35(10):792‐5. - PubMed
Pita Martin 1999 {published and unpublished data}
-
- Pita Martin de Portela ML. Personal communication 2004 March 22.
-
- Pita Martin de Portela ML, Langini SH, Fleischman S, Garcia M, Lopez LB, Guntin R, et al. Effect of iron supplementation and its frequency during pregnancy. Medicina 1999;59:430‐6. - PubMed
Powers 1985 {published data only}
-
- Powers HJ, Bates CJ, Lamb WH. Haematological response to supplements of iron and riboflavin to pregnant and lactating women in rural Gambia. Human Nutrition. Clinical Nutrition 1985;39(2):117‐29. - PubMed
Quintero 2004 {unpublished data only}
-
- Quintero Gutierrez AG, Gonzalez Rosendo G, Cedillo Espana F, Rivera‐Dommarco J. Single weekly iron supplementation in pregnant women. Personal communication 2004 February 17.
Rae 1970 {published data only}
Ramakrishnan 2003 {published data only}
-
- Ramakrishnan U, Gonzalez‐Cossio T, Neufeld LM, Rivera J, Martorell R. Multiple micronutrient supplementation during pregnancy does not lead to greater infant birth size than does iron‐only supplementation: a randomized controlled trial in a semirural community in Mexico. American Journal of Clinical Nutrition 2003;77(3):720‐5. - PubMed
Rayado 1997 {published data only}
-
- Rayado B, Carrillo JA, Fernandez‐Esteban JA, Gomez‐Cedillo A, Martin M, Coronel P. A comparative study of 2 ferrous proteins in the prevention of iron deficiency anaemia during pregnancy. Clinica e Investigacion en Ginecologia y Obstetricia 1997;24:46‐50.
Reddaiah 1989 {published data only}
-
- Reddaiah VP, Raj PP, Ramachandran K, Nath LM, Sood SK, Madan N, et al. Supplementary iron dose in pregnancy anemia prophylaxis. Indian Journal of Pediatrics 1989;56:109‐14. - PubMed
Ridwan 1996 {published and unpublished data}
-
- Ridwan E, Schultink W, Dillon D, Gross R. Effects of weekly iron supplementation on pregnant Indonesian women are similar to those of daily supplementation. American Journal of Clinical Nutrition 1996;63(6):884‐90. - PubMed
-
- Schultink W, Ridwan E, Dillon D, Gross R. Individual patient data (as supplied 12 January 2004). Data on file.
Robinson 1998 {published and unpublished data}
-
- Robinson JS. Individual patient data (as supplied 11 March 2004). Data on file.
-
- Robinson JS. Working with traditional birth attendants to improve iron tablet utilization by pregnant women. MotherCare Technical Working Paper #7. Arlington, VA 1998.
-
- Robinson JS, Sopacua J, Napitapulu J. Using traditional birth attendants to improve iron tablet utilization by pregnant women. Maluku Province, Indonesia. Draft paper. Mother Care Project. Project Concern International San Diego CA 1999.
-
- Robinson JS, Yip R. Weekly versus daily iron tablet supplementation in pregnant women in Indonesia. Draft paper 2000.
Rolschau 1979 {published data only}
-
- Rolschau J, Date J, Kristoffersen K. Folic acid supplement and intrauterine growth. Acta Obstetricia et GynecologicaScandinavica 1979;58:343‐6. - PubMed
Roth 1980 {published data only}
-
- Roth F, Mauracher E. Folic acid treatment during pregnancy [Folsauresubstitution bei schwangeren]. Geburtshilfe und Frauenheilkunde 1980;40:253‐8. - PubMed
Roztocil 1994 {published data only}
-
- Roztocil A, Charvatova M, Harastova L, Zahradkova J, Studenik P, Sochorova V, et al. Anti‐anemia therapy with prophylactic administration of fe2+ in normal pregnancy and its effect on prepartum hematologic parameters in the mother and neonate. Ceska Gynekologie 1994;59(3):130‐3. - PubMed
Rukhsana 2006 {published data only}
-
- Rukhsana N, Bano H, Gurbakhshani AL, Dudani AL, Jafri TK, Mannan A. Changes in hemoglobin, red cell count, red cell indices and reticulocyte count in response to daily versus intermittent oral iron supplementation during pregnancy in local population. Medical Channel 2006;12(3):7‐10.
Rybo 1971 {published data only}
-
- Rybo G, Solvell L. Side‐effect studies on a new sustained release iron preparation. Scandinavian Journal of Hematology 1971;8(4):257‐64. - PubMed
Sachdeva 1993 {published data only}
-
- Sachdeva R, Mann SK. Impact of nutrition education and medical supervision on pregnancy outcome. Indian Pediatrics 1993;30(11):1309‐14. - PubMed
Saha 2007 {published data only}
Sandstad 2003 {published data only}
-
- Sandstad B, Borch‐Iohnson B, Andersen GM, Dahl‐Jorgensen B, Froysa I, Leslie C, et al. Selective iron supplementation based on serum ferritin values early in pregnancy: are the Norwegian recommendations satisfactory?. Acta Obstetricia et Gynecologica Scandinavica 2003;82:537‐42. - PubMed
Schoorl 2012 {published data only}
Seck 2008 {published data only}
-
- Seck BC, Jackson RT. Determinants of compliance with iron supplementation among pregnant women in Senegal. Public Health Nutrition 2008;11(6):596‐605. - PubMed
-
- Seck BC, Jackson RT. Providing iron/folic acid tablets free of charge improves compliance in pregnant women in Senegal. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103(5):485‐92. - PubMed
Shatrugna 1999 {published data only}
-
- Shatrugna V, Raman L, Kailash U, Balakrishna N, Rao KV. Effect of dose and formulation on iron tolerance in pregnancy. National Medical Journal of India 1999;12(1):18‐20. - PubMed
Sinha 2011 {published data only}
-
- Sinha V, Dayal M, Mehrotra R, Mishra V. Intravenous iron sucrose versus oral ferrous ascorbate in the prevention of anaemia in pregnant women. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:72.
Sjostedt 1977 {published data only}
-
- Sjostedt JE, Manner P, Nummi S, Ekenved G. Oral iron prophylaxis during pregnancy ‐ a comparative study on different dosage regimens. Acta Obstetricia et Gynecologica Scandinavica 1977;66:3‐9. - PubMed
Sood 1979 {published data only}
-
- Sood SK, Ramachandran K, Rani K, Ramalingaswami V, Mathan VI, Ponniah J, et al. WHO sponsored collaborative studies on nutritional anaemia in India. The effect of parenteral iron administration in the control of anaemia of pregnancy. British Journal of Nutrition 1979;42:399‐406. - PubMed
Srisupandit 1983 {published data only}
-
- Srisupandit S, Pootrakul P, Areekul S, Neungton S, Mokkaves J, Kiriwat O, et al. A prophylactic supplementation of iron and folate in pregnancy. Southeast Asian Journal of Tropical Medicine and Public Health 1983;14(3):317‐23. - PubMed
Steer 1992 {published data only}
-
- Steer PJ. Trial to assess the effects of iron and folate supplementation on pregnancy outcome [trial abandoned]. Personal communication 1992.
Stone 1975 {published data only}
-
- Stone M, Elder MG. The relative merits of a slow‐release and a standard iron preparation during pregnancy. Current Medical Research and Opinion 1975;3:469‐72.
Swain 2011 {published data only}
-
- Swain S, Mahapatra PC, Majhi C, Das TK. Two doses of parenteral iron sucrose injection as anaemia prophylaxis in pregnancy. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:181.
Tampakoudis 1996 {published data only}
-
- Tampakoudis P, Tantanassis T, Tsatalas K, Lazaridis E, Tsalikis T, Venetis C, et al. A randomized trial on the effect of oral supplementation with iron protein succinylate in singleton pregnancies. The role of maternal erythropoietin as a marker. Prenatal and Neonatal Medicine 1996;1 Suppl 1:181.
Tan 1995 {published data only}
-
- Tan CH, Ng KB. The effect of oral iron on the haemoglobin concentration during the second half of pregnancy. 27th British Congress of Obstetrics and Gynaecology 1995 July 4‐7; Dublin, Ireland. Royal College of Obstetricians & Gynaecologists, 1995:101.
Tange 1993 {published data only}
-
- Tange E, Weigand E, Mbofung CM. Effect of iron supplementation on anemic and non‐anemic pregnant teenagers in Cameroon. TEMA 8: Proceedings of the Eighth International Symposium on Trace Elements in Man and Animals; 1993 May 16‐22; Dresden, Germany. 1993:220‐3.
Thane‐Toe 1982 {published data only}
-
- Thane‐Toe, Thein‐Than. The effects of oral iron supplementation on ferritin levels in pregnant Burmese women. American Journal of Clinical Nutrition 1982;35(1):95‐9. - PubMed
Thomsen 1993 {published data only}
-
- Thomsen JK, Prien‐Larsen JC, Devantier A, Fogh‐Andersen N. Low dose iron supplementation does not cover the need for iron during pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1993;72:93‐8. - PubMed
Trigg 1976 {published data only}
Vogel 1963 {published data only}
-
- Vogel L, Steingold L, Suchet J. Iron therapy in the treatment of anaemia in pregnancy. Lancet 1963;1:1296‐9. - PubMed
Wali 2002 {published data only}
-
- Wali A, Mushtaq A, Nilofer. Comparative study‐‐efficacy, safety and compliance of intravenous iron sucrose and intramuscular iron sorbitol in iron deficiency anemia of pregnancy. Journal of the Pakistan Medical Association 2002;52(9):392‐5. - PubMed
Weil 1977 {published data only}
-
- Weil A, Mauracher E. Folic acid and pregnancy, a real problem? [Acide folique et gravidité, problème réel?]. Schweizer Medizinische Wochenschrift 1977;107(52):1943‐7. - PubMed
West 2014 {published data only}
-
- West K, Shamim A, Mehra S, Labrique A, Ali H, Shaikh S, et al. Effect of maternal multiple micronutrient vs iron–folic acid supplementation on infant mortality and adverse birth outcomes in rural Bangladesh. The JiVitA‐3 randomized trial. JAMA 2014;312(24):2649‐58. [DOI: 10.1001/jama.2014.16819] - DOI - PubMed
Willoughby 1966 {published data only}
Willoughby 1968 {published data only}
Winichagoon 2003 {unpublished data only}
-
- Winichagoon P, Lertmullikaporn N, Chitcumroonchokechai C, Thamrongwarangkul T. Daily versus weekly iron supplementation to pregnant women in rural northeast Thailand. Personal communication 2003.
Wu 1998 {published data only}
-
- Wu Y, Weng L, Wu L. Clinical experience with iron supplementation in pregnancy. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 1998;33(4):206‐8. - PubMed
Yecta 2011 {published data only}
Young 2000 {published data only}
-
- Young MW, Lupafya E, Kapenda E, Bobrow EA. The effectiveness of weekly iron supplementation in pregnant women of rural northern Malawi. Tropical Doctor 2000;30(2):84‐8. - PubMed
Young 2010 {published data only}
Yu 1998 {published and unpublished data}
-
- Yu KH, Yoon JS. The effect of weekly iron supplementation on iron and zinc nutritional status in pregnant women. Korean Journal of Nutrition 1998;31(8):1270‐82.
-
- Yu KH, Yoon, JS. Individual patient data (as supplied 11 March 2004). Data on file.
Zamani 2008 {published data only}
-
- Zamani AR, Farajzadegan Z, Ghahiri A, Khademloo M, Golshiri P. Effectiveness of twice weekly iron supplementation compared with daily regimen in reducing anemia and iron deficiency during pregnancy: a randomized trial in Iran. Journal of Research in Medical Sciences 2008;13(5):230‐9.
Zhou 2009 {published data only}
-
- Zhou SJ, Gibson RA, Crowther CA, Makrides M. Should we lower the dose of iron when treating anaemia in pregnancy? A randomized dose‐response trial. European Journal of Clinical Nutrition 2009;63(2):183‐90. - PubMed
Zutschi 2004 {published data only}
-
- Zutschi V, Batra S, Ahmad SS, Khera N, Chauhan G, Gandhi G, et al. Injectable iron supplementation instead of oral therapy for antenatal care. Journal of Obstetrics and Gynecology of India 2004;54(1):37‐8.
References to studies awaiting assessment
Parisi 2013 {published data only}
-
- Parisi F, Fuse F, Brunetti M, Mazzocco M, Berti C, Cetin I. Effects of different regimens of iron supplementation on iron status and pregnancy outcomes in a cohort of healthy pregnant women: a randomized control trial. Journal of Perinatal Medicine 2013;41(Suppl 1):Abstract no:595.
References to ongoing studies
Dibley 2012 {published data only}
-
- A trial to evaluate the impact of an early start to iron/folic acid supplementation in pregnancy on deaths of newborns in rural Bangladesh. Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) (accessed 2 July 2012) 2012.
Fawzi 2010 {published data only}
-
- Fawzi W. Prenatal iron supplements: safety and efficacy in Tanzania. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 12 November 2010) 2010.
Hamzehgardeshi 2009 {published data only}
-
- Hamzehgardeshi Z. A randomized placebo‐controlled trial to determine the effect of iron supplementation on hematological indices in pregnant women with hemoglobin ≥13.2 g/dl. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010) 2010.
Jafarbegloo 2010 {published data only}
-
- Jafarbegloo E. Gastrointestinal complications of iron supplement in pregnant women. IRCT Iranian Registry of Clinical Trials (www.irct.ir) (accessed 6 December 2010) 2010.
Mwangi 2011 {published data only}
-
- Mwangi MN. A randomised trial to assess the safety and efficacy or iron supplementation in Kenyan pregnant women. ClinicalTrials.gov (http://clinicaltrials.gov) (accessed 11 March 2011) 2011.
-
- Mwangi MN, Andang'o PEA, Mwangi AM, Verhoef H, Savelkoul H. Safety and efficacy of fortification versus fortification plus supplementation with iron in African pregnant women: A randomised controlled trial. Annals of Nutrition and Metabolism 2013;63(Suppl):1162‐1163.
Ramakrishnan 2012 {published data only}
-
- Ramakrishnan U. Impact of pre‐pregnancy micronutrient supplementation on maternal and child outcomes. http://clinicaltrials.gov/ct2/show/record/NCT01665378 (accessed 20 September 2012) 2012.
Zhao 2014 {published data only}
-
- Zhao G. Impact of iron/folic acid versus folic acid supplements during pregnancy on maternal and children's health: a randomized controlled trial in China. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 27 August 2014] 2014.
Additional references
Andersen 2006
-
- Andersen HS, Gambling L, Holtrop G, McArdle HJ. Maternal iron deficiency identifies critical windows for growth and cardiovascular development in the rat postimplantation embryo. Journal of Nutrition 2006;136(5):1171‐7. - PubMed
Balshem 2010
-
- Balshem H, Helfanda M, Schunemann HJ, Oxman AD, Kunze R, Brozek J, et al. GRADE guidelines: rating the quality of evidence: introduction. Journal of Clinical Epidemiology 2010;64(4):401‐6. - PubMed
BCSH 2011
-
- British Committee for Standards in Haematology. UK guidelines on the management of iron deficiency in pregnancy. Vol. (http://www.bcshguidelines.com/documents/UK_Guidelines_iron_deficiency_in..., accessed on 18 May 2015), London: British Society for Haematology, 2011.
Beard 2000
-
- Beard J. Effectiveness and strategies of iron supplementation during pregnancy. American Journal of Clinical Nutrition 2000;71(5):1288S‐1294S. - PubMed
Beaton 1999
-
- Beaton GH, McCabe G. Efficacy of intermittent iron supplementation in the control of iron deficiency anaemia in developing countries. An analysis of experience. Ottawa: The Micronutrient Initiative, 1999.
Beaton 2000
-
- Beaton GH. Iron needs during pregnancy: do we need to rethink our targets?. American Journal of Clinical Nutrition 2000;72(1 Suppl):265S‐271S. - PubMed
Bhutta 2008
-
- Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet 2008;371(9610):417‐40. - PubMed
Black 2011
-
- Black M, Quigg A, Hurley K, Pepper M. Iron deficiency and iron‐deficiency anemia in the first two years of life: strategies to prevent loss of developmental potential. Nutrition Reviews 2011;69(Suppl 1):S64‐S70. - PubMed
Bothwell 1981
-
- Bothwell TH, Charlton RW, editors. Iron Deficiency in Women. Washington DC: Nutrition Foundation, 1981.
Bothwell 2000
-
- Bothwell TH. Iron requirements in pregnancy and strategies to meet them. American Journal of Clinical Nutrition 2000;72(1 Suppl):257S‐264S. - PubMed
Burke 2014
Cantor 2015
-
- Cantor AG, Bougatsos C, Dana T, Blazina I, McDonagh M. Routine iron supplementation and screening for iron deficiency anemia in pregnancy: a systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2015;162(8):566‐76. - PubMed
CDC 1998
-
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. Morbidity and Mortality Weekly Report 1998;47(RR‐3):1‐29. - PubMed
Christian 2010
-
- Christian P. Micronutrients, birth weight, and survival. Annual Review of Nutrition 2010;30:83‐104. - PubMed
Cook 2003
-
- Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood 2003;101(9):3359‐64. - PubMed
Crompton 2002
-
- Crompton DW, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annual Review of Nutrition 2002;22:35‐59. - PubMed
De‐Regil 2010
Garcia‐Casal 2014
-
- Garcia‐Casal MN, Peña‐Rosas JP, Pasricha SR. Rethinking ferritin cutoffs for iron deficiency and overload. Lancet Hematology 2014;1:e92‐e94. - PubMed
Garn 1981
-
- Garn SM, Ridella SA, Petzold AS, Falkner F. Maternal hematologic levels and pregnancy outcomes. Seminars in Perinatology 1981;5:155‐62. - PubMed
Gleason 2007
-
- Gleason G, Scrimshaw NS. An overview of the functional significance if iron deficiency. In: Kraemer K, Zimmermann MB editor(s). Nutritional Anemia. Vol. 1, Basel, Switzerland: Sight and Life, 2007:45‐58.
Godfrey 1991
-
- Godfrey KM, Redman CW, Barker DJ, Osmond C. The effect of maternal anaemia and iron deficiency on the ratio of fetal weight to placental weight. British Journal of Obstetrics and Gynaecology 1991;98(9):886‐91. - PubMed
GRADEpro 2014 [Computer program]
-
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2015. McMaster University, 2014.
Haider 2012
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hunt 2002
-
- Hunt JM. Reversing productivity losses from iron deficiency: the economic case. Journal of Nutrition 2002;132(4 Suppl):794S‐801S. - PubMed
Hytten 1964
-
- Hytten FE, Leitch I. The Physiology of Human Pregnancy. Oxford: Blackwell Scientific Publications, 1964:14.
Hytten 1971
-
- Hytten FE, Leitch I, Baird D. The Physiology of Human Pregnancy. 2nd Edition. Oxford: Blackwell Scientific Publications, 1971.
Imdad 2012
-
- Imdad A, Bhutta ZA. Routine iron/folate supplementation during pregnancy: effect on maternal anaemia and birth outcomes. Paediatric and Perinatal Epidemiology 2012;26 (Suppl. 1):168–77. - PubMed
INACG 1998
-
- Stoltzfus R, Dreyfuss M. Guidelines for the use of iron supplements to prevent and treat iron deficiency anaemia. Washington DC: ILSI Press, 1998.
INACG 2002a
-
- International Nutritional Anemia Consultative Group. Anemia, iron deficiency and iron deficiency anemia. INACG, 2002.
INACG 2002b
-
- International Nutritional Anemia Consultative Group (INACG). Why is iron important and what to do about it: a new perspective. Report of the 2001 INACG Symposium; 2001 February 15‐16; Hanoi, Vietnam. 2002:1‐50.
IOM 2001
-
- Institute of Medicine. Iron. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington DC: National Academy Press, 2001:290‐393. - PubMed
Kandoi 1991
-
- Kandoi A, Bhatia BD, Pandey LK, Pandey S, Sen PC, Satya K. Cellular immunity status in anaemia in pregnancy. Indian Journal of Medical Research 1991;94:11‐5. - PubMed
Kim 1992
-
- Kim I, Hungerford DW, Yip R, Kuester SA, Zyrkowski C, Trowbridge FL. Pregnancy nutrition surveillance system‐‐United States, 1979‐1990. Morbidity and Mortality Weekly Report 1992;41(7):25‐41. - PubMed
Klebanoff 1989
-
- Klebanoff MA, Shiono PH, Berendes HW, Rhoads GG. Facts and artifacts about anemia and preterm delivery. JAMA 1989;262(4):511‐5. - PubMed
Klebanoff 1991
-
- Klebanoff MA, Shiono PH, Selby JV, Trachtenberg AI, Graubard BI. Anemia and spontaneous preterm birth. American Journal of Obstetrics and Gynecology 1991;164(1):59‐63. - PubMed
Langendam 2013
Lassi 2013
Lozoff 2006
Lutter 2011
-
- Lutter CK, Daelmans BM, Onis M, Kothari MT, Ruel MT, Arimond M, et al. Undernutrition, poor feeding practices, and low coverage of key nutrition interventions. Pediatrics 2011;128(6):e1418‐e1427. - PubMed
Mahomed 1997
McDonald 2013
Mei 2011
-
- Mei Z, Cogswell ME, Looker AC, Pfeiffer CM, Cusick SE, Lacher DA, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999‐2006. American Journal of Clinical Nutrition 2011;93(6):1312‐20. - PubMed
Milman 2007
-
- Milman N, Bergholt T, Byg KE, Eriksen L, Hvas AM. Reference intervals for haematological variables during normal pregnancy and postpartum in 434 healthy Danish women. European Journal of Haematology 2007;79(1):39‐46. - PubMed
Mora 2002
-
- Mora JO. Iron supplementation: overcoming technical and practical barriers. Journal of Nutrition 2002;132(4 Suppl):853S‐855S. - PubMed
Murphy 1986
-
- Murphy JF, O'Riordan J, Newcombe RG, Coles EC, Pearson JF. Relation of haemoglobin levels in first and second trimesters to outcome. Lancet 1986;3(1):992‐5. - PubMed
Nagata 2011
Nair 2004
-
- Nair KM, Bhaskaram P, Balakrishna N, Ravinder P, Sesikeran B. Response of hemoglobin, serum ferritin, and serum transferrin receptor during iron supplementation in pregnancy: a prospective study. Nutrition 2004;20(10):896‐9. - PubMed
Nel 2015
NIH 2011
-
- NIH Iron and Malaria Technical Working Group. Chapter 2: Mechanisms. In: Raiten D, Namaste S, Brabin B editor(s). Considerations for the Safe and Effective Use of Iron Interventions. Bethesda: Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), 2011 (in press):16‐51.
Nisar 2014
Oppenheimer 2001
-
- Oppenheimer SJ. Iron and its relation to immunity and infectious disease. Journal of Nutrition 2001;131:616S‐635S. - PubMed
Parker 2012
Pasricha 2014
-
- Pasricha SR, Low M, Thompson J, Farrell A, De‐Regil LM. Iron supplementation benefits physical performance in women of reproductive age: a systematic review and meta‐analysis. Journal of Nutrition 2014;144(6):906‐14. - PubMed
Peña‐Rosas 2012a
Prema 1982
-
- Prema K, Ramalakshmi BA, Madhavapeddi R, Babu S. Immune status of anaemic pregnant women. British Journal of Obstetrics and Gynaecology 1982;89:222‐5. - PubMed
Rabe 2012
-
- Rabe H, Diaz‐Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD003248.pub3] - DOI - PubMed
Ramakrishnan 2013
-
- Ramakrishnan U, Grant FK, Imdad A, Bhutta ZA, Martorell R. Effect of multiple micronutrient versus iron‐folate supplementation during pregnancy on intrauterine growth. Nestle Nutrition Institute Workshop Series 2013;74:53‐62. - PubMed
Reveiz 2011
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sanghvi 2010
-
- Sanghvi T, Harvey P, Wainwright E. Maternal iron‐folic acid supplementation programs: evidence of impact and implementation. Food & Nutrition Bulletin 2 2010;31(Suppl 2):S100‐S107. - PubMed
Scholl 1992
-
- Scholl TO, Hediger, ML, Fischer RL, Shearer JW. Anaemia vs. iron deficiency: increased risk of preterm delivery in a prospective study. American Journal of Clinical Nutrition 1992;55:985‐8. - PubMed
Scholl 1997
-
- Scholl TO, Hediger ML, Bendich A, Schall JI, Smith WK, Krueger PM. Use of multivitamin/mineral prenatal supplements: influence on the outcome of pregnancy. American Journal of Epidemiology 1997;146:134‐41. - PubMed
Scholl 1998
-
- Scholl TO. High third‐trimester ferritin concentration: associations with very preterm delivery, infection, and maternal nutritional status. Obstetrics & Gynecology 1998;92:161‐6. - PubMed
Scholl 2000
-
- Scholl TO, Reilly T. Anemia, iron and pregnancy outcome. Journal of Nutrition 2000;130(Suppl 2):443S‐447S. - PubMed
Scholl 2005
-
- Scholl TO. Iron status during pregnancy: setting the stage for mother and infant. American Journal of Clinical Nutrition 2005;81(5):1218S‐1222S. - PubMed
Schümann 2007
-
- Schümann K, Ettle T, Szegner B, Elsenhans B, Solomons NW. On risks and benefits of iron supplementation recommendations for iron intake revisited. Journal of Trace Elements in Medicine and Biology 2007;21(3):147‐68. - PubMed
Shrimptom 2009
-
- Shrimpton R, Huffman SL, Zehner ER, Darnton‐Hill I, Dalmiya N. Multiple micronutrient supplementation during pregnancy in developing‐country settings: policy and program implications of the results of a meta‐analysis. Food and Nutrition Bulletin 2009;30(4Suppl):S556‐S573. - PubMed
Sloan 2002
Steer 2000
-
- Steer PJ. Maternal hemoglobin concentration and birth weight. American Journal of Clinical Nutrition 2000;71(5 Suppl):1285S‐1287S. - PubMed
Van den Broek 2010
Villar 1997
-
- Villar J, Bergsjo P. Scientific basis for the content of routine antenatal care. I. Philosophy, recent studies and power to eliminate or alleviate adverse maternal outcomes. Acta Obstetricia et Gynecologica Scandinavica 1997;76(1):1‐14. - PubMed
Villar 2003
-
- Villar J, Merialdi M, Gulmezoglu AM, Abalos E, Carroli G, Kulier R, et al. Nutritional interventions during pregnancy for the prevention or treatment of maternal morbidity and preterm delivery: an overview of randomized controlled trials. Journal of Nutrition 2003;5(Suppl 2):1606S‐1625S. - PubMed
WHO 1959
-
- World Health Organization. Iron Deficiency Anaemias: Report of a WHO study group. WHO Technical Report Series #182. Geneva: World Health Organization, 1959. - PubMed
WHO 1968
-
- World Health Organization. Nutritional Anaemias: Report of a WHO Scientific Group: WHO Technical Report Series #405. Geneva: World Health Organization, 1968. - PubMed
WHO 1992
-
- World Health Organization. The Prevalence of Anaemia in Women: a Tabulation of Available Information. 2nd Edition. Geneva: World Health Organization, 1992.
WHO 2001
-
- World Health Organization. Iron Deficiency Anaemia Assessment Prevention and Control: a Guide For Program Managers. Geneva: World Health Organization, 2001.
WHO 2008
-
- World Health Organization. The International Pharmacopoeia. (http://apps.who.int/phint/en/p/about/) (accessed 2008) 2008.
WHO 2010
-
- World Health Organization. Malaria. In: Poumerol G, Wilder‐Smith A editor(s). Malaria. International Travel and Health. Situation as on 1 January 2010. Geneva: World Health Organization, 2010.
WHO 2011a
-
- World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. http://www.who.int/iris/bitstream/10665/85839/http://apps.who.int//iris/... (accessed 18 May 2015) 2011.
WHO 2011b
-
- World Health Organization. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Vitamin and Mineral Nutrition Information System (WHO/NMH/NHD/MNM/11.2) (http://www.who.int/vmnis/indicators/ferritin/en/index.html (accessed April 29 2011) 2011.
WHO 2011c
-
- World Health Organization. E‐book International Travel and Health 2011 (PDF format). Geneva: World Health Organization, 2011.
WHO 2012a
-
- World Health Organization. Guideline: daily iron and folic acid supplementation in pregnant women. (http://apps.who.int/iris/bitstream/10665/77770/1/9789241501996_eng.pdf, accessed 18 May 2015) 2012. - PubMed
WHO 2012b
-
- World Health Organization. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva: World Health Organization, 2012. - PubMed
WHO 2014a
-
- World Health Organization. Serum transferrin receptor levels for the assessment of iron status and iron deficiency in populations. Vitamin and Mineral Nutrition Information System (WHO/NMH/NHD/MNM/14.6). (http://apps.who.int/iris/bitstream/10665/133707/1/WHO_NMH_NHD_EPG_14.6_e..., accessed 18 May 2015) 2014.
WHO 2014b
-
- World Health Organization. Guideline: delayed umbilical cord clamping for improved maternal and infant health and nutrition outcomes. (http://www.who.int/iris/bitstream/10665/148793/http://apps.who.int//iris..., accessed 18 May 2015) 2014. - PubMed
WHO 2014c
-
- World Health Organization. World Malaria Report 2014. (http://apps.who.int/iris/bitstream/10665/144852/2/9789241564830_eng.pdf, accessed 18 May 2015) 2014.
WHO 2015a
-
- World Health Organization. Serum and red blood cell folate concentrations for assessing folate status in populations. Vitamin and Mineral Nutrition Information System (WHO/NMH/NHD/EPG/15.01). (http://www.who.int/iris/bitstream/10665/162114/http://apps.who.int//iris..., accessed 18 May 2015) 2015.
WHO 2015b
-
- World Health Organization. Global prevalence of anaemia in 2011. Geneva: World Health Organization, 2015.
WHO/CDC 2005
-
- World Health Organization, Centers for Disease Control and Prevention. Assessing the iron status of populations. Report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level. Geneva, Switzerland: World Health Organization and Centers for Disease Control and Prevention, 2005:1‐30.
Xiong 2000
-
- Xiong X, Buekens P, Alexander S, Demianczuk N, Wollast E. Anemia during pregnancy and birth outcome: a meta‐analysis. American Journal of Perinatology 2000;17(3):137‐46. - PubMed
Yakoob 2011
Zhou 1998
-
- Zhou LM, Yang WW, Hua JZ, Deng CQ, Tao X, Stoltzfus RJ. Relation of hemoglobin measured at different times in pregnancy to preterm birth and low birth weight in Shanghai, China. American Journal of Epidemiology 1998;148(10):998‐1006. - PubMed
References to other published versions of this review
Mahomed 1998b
Mahomed 2000a
Mahomed 2006a
Mahomed 2006b
Peña‐Rosas 2004
Peña‐Rosas 2006
Peña‐Rosas 2009
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

