Efficacy of galactose and adalimumab in patients with resistant focal segmental glomerulosclerosis: report of the font clinical trial group
- PMID: 26198842
- PMCID: PMC4511259
- DOI: 10.1186/s12882-015-0094-5
Efficacy of galactose and adalimumab in patients with resistant focal segmental glomerulosclerosis: report of the font clinical trial group
Abstract
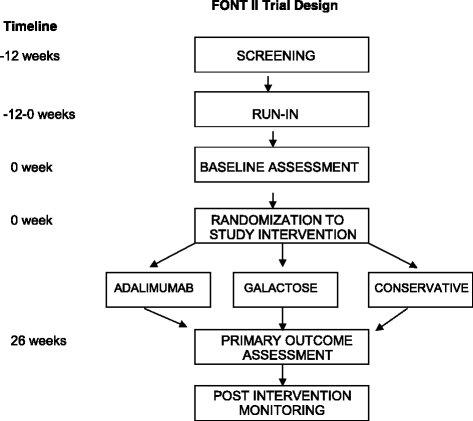
Background: Patients with resistant focal segmental glomerulosclerosis (FSGS) who are unresponsive to corticosteroids and other immunosuppressive agents are at very high risk of progression to end stage kidney disease. In the absence of curative treatment, current therapy centers on renoprotective interventions that reduce proteinuria and fibrosis. The FONT (Novel Therapies for Resistant FSGS) Phase II clinical trial (NCT00814255, Registration date December 22, 2008) was designed to assess the efficacy of adalimumab and galactose compared to standard medical therapy which was comprised of lisinopril, losartan, and atorvastatin.
Methods: Key eligibility criteria were biopsy confirmed primary FSGS or documentation of a causative genetic mutation, urine protein:creatinine ratio >1.0 g/g, and estimated glomerular filtration rate (eGFR) >40 ml/min/1.73 m(2). The experimental treatments - adalimumab, galactose, standard medical therapy-- were administered for 26 weeks. The primary endpoint was a 50 % reduction in proteinuria with stable eGFR.
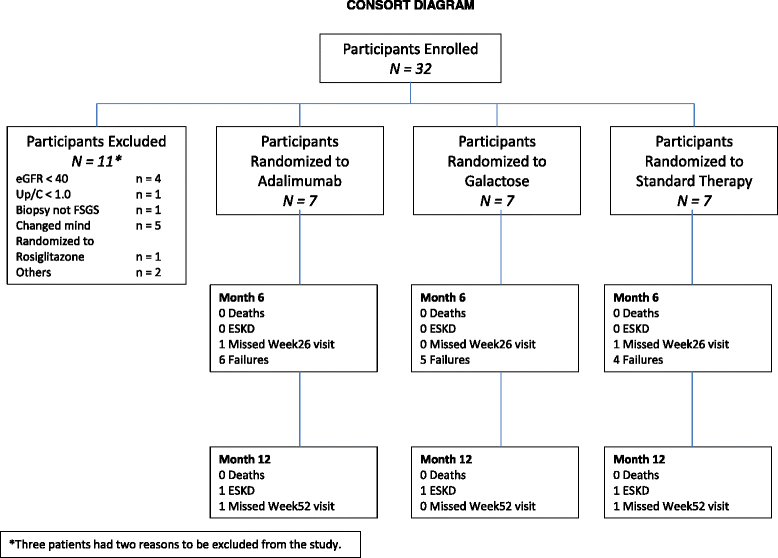
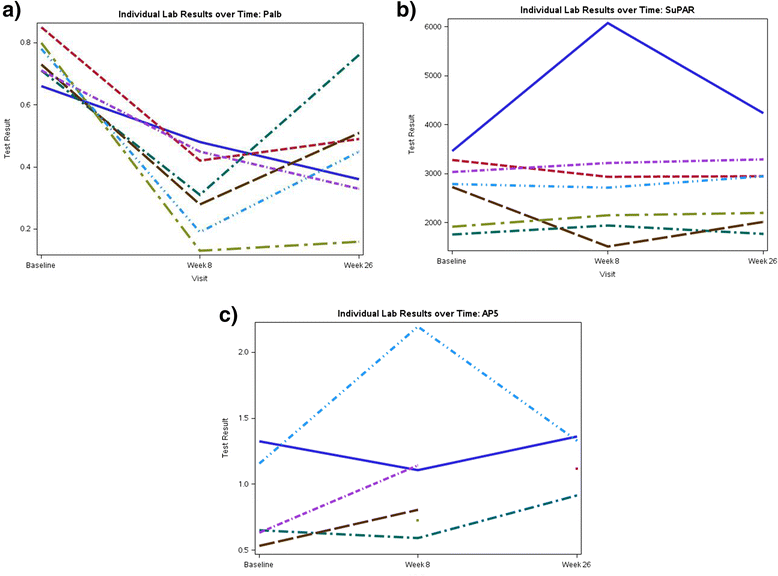
Results: Thirty-two subjects were screened and 21 were assigned to one of the three study arms. While none of the adalimumab-treated subjects achieved the primary outcome, 2 subjects in the galactose and 2 in the standard medical therapy arm had a 50 % reduction in proteinuria without a decline in eGFR. The proteinuria response did not correlate with serial changes in the serum glomerular permeability activity measured by the Palb assay or soluble urokinase plasminogen activator receptor (suPAR). There were no serious adverse effects related to treatments in the study.
Conclusions: Recruitment into this trial that addressed patients with resistant FSGS fell short of the enrollment goal. Our findings suggest that future studies of novel therapies for rare glomerular diseases such as FSGS may benefit from enrollment of patients earlier in the course of their disease. In addition, better identification of patients who are likely to respond to a new treatment based on biomarkers suggesting involvement of the disease pathway targeted by the experimental agent may reduce the required sample size and increase the likelihood of a favorable outcome.
Figures

References
-
- Sadowski CE, Lovric S, Ashraf S, Pabst WL, Gee HY, Kohl S, et al; the SRNS Study Group, Hildebrandt F: A single-gene cause in 29.5 % of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol 2014 Oct 27. pii: ASN.2014050489. [Epub ahead of print] - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

