A role for the mevalonate pathway in early plant symbiotic signaling
- PMID: 26199419
- PMCID: PMC4534228
- DOI: 10.1073/pnas.1413762112
A role for the mevalonate pathway in early plant symbiotic signaling
Erratum in
-
Correction for Venkateshwaran et al., A role for the mevalonate pathway in early plant symbiotic signaling.Proc Natl Acad Sci U S A. 2015 Sep 22;112(38):E5378. doi: 10.1073/pnas.1516711112. Epub 2015 Aug 31. Proc Natl Acad Sci U S A. 2015. PMID: 26324931 Free PMC article. No abstract available.
Abstract
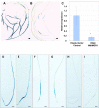
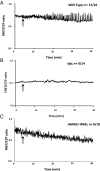
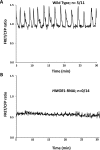
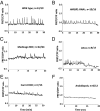
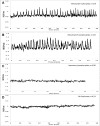
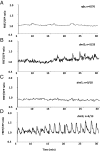
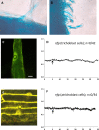
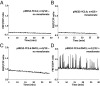
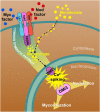
Rhizobia and arbuscular mycorrhizal fungi produce signals that are perceived by host legume receptors at the plasma membrane and trigger sustained oscillations of the nuclear and perinuclear Ca(2+) concentration (Ca(2+) spiking), which in turn leads to gene expression and downstream symbiotic responses. The activation of Ca(2+) spiking requires the plasma membrane-localized receptor-like kinase Does not Make Infections 2 (DMI2) as well as the nuclear cation channel DMI1. A key enzyme regulating the mevalonate (MVA) pathway, 3-Hydroxy-3-Methylglutaryl CoA Reductase 1 (HMGR1), interacts with DMI2 and is required for the legume-rhizobium symbiosis. Here, we show that HMGR1 is required to initiate Ca(2+) spiking and symbiotic gene expression in Medicago truncatula roots in response to rhizobial and arbuscular mycorrhizal fungal signals. Furthermore, MVA, the direct product of HMGR1 activity, is sufficient to induce nuclear-associated Ca(2+) spiking and symbiotic gene expression in both wild-type plants and dmi2 mutants, but interestingly not in dmi1 mutants. Finally, MVA induced Ca(2+) spiking in Human Embryonic Kidney 293 cells expressing DMI1. This demonstrates that the nuclear cation channel DMI1 is sufficient to support MVA-induced Ca(2+) spiking in this heterologous system.
Keywords: HMG-CoA reductase; arbuscular mycorrhization; calcium signaling; legume nodulation; mevalonate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Osbourn A. Saponins and plant defence—A soap story. Trends Plant Sci. 1996;1(1):4–9.
-
- Venkateshwaran M, Volkening JD, Sussman MR, Ané JM. Symbiosis and the social network of higher plants. Curr Opin Plant Biol. 2013;16(1):118–127. - PubMed
-
- Amor BB, et al. The NFP locus of Medicago truncatula controls an early step of Nod factor signal transduction upstream of a rapid calcium flux and root hair deformation. Plant J. 2003;34(4):495–506. - PubMed
-
- Fliegmann J, et al. Lipo-chitooligosaccharidic symbiotic signals are recognized by LysM receptor-like kinase LYR3 in the legume Medicago truncatula. ACS Chem Biol. 2013;8(9):1900–1906. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

