Inhibition of kinesin-5 improves regeneration of injured axons by a novel microtubule-based mechanism
- PMID: 26199587
- PMCID: PMC4498332
- DOI: 10.4103/1673-5374.158351
Inhibition of kinesin-5 improves regeneration of injured axons by a novel microtubule-based mechanism
Abstract
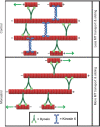
Microtubules have been identified as a powerful target for augmenting regeneration of injured adult axons in the central nervous system. Drugs that stabilize microtubules have shown some promise, but there are concerns that abnormally stabilizing microtubules may have only limited benefits for regeneration, while at the same time may be detrimental to the normal work that microtubules perform for the axon. Kinesin-5 (also called kif11 or Eg5), a molecular motor protein best known for its crucial role in mitosis, acts as a brake on microtubule movements by other motor proteins in the axon. Drugs that inhibit kinesin-5, originally developed to treat cancer, result in greater mobility of microtubules in the axon and an overall shift in the forces on the microtubule array. As a result, the axon grows faster, retracts less, and more readily enters environments that are inhibitory to axonal regeneration. Thus, drugs that inhibit kinesin-5 offer a novel microtubule-based means to boost axonal regeneration without the concerns that accompany abnormal stabilization of the microtubule array. Even so, inhibiting kinesin-5 is not without its own caveats, such as potential problems with navigation of the regenerating axon to its target, as well as morphological effects on dendrites that could affect learning and memory if the drugs reach the brain.
Keywords: Eg5; axon; kinesin-5; microtubule; molecular motor protein; monastrol; regeneration.
Figures

References
-
- Ahmad FJ, He Y, Myers KA, Hasaka TP, Francis F, Black MM, Baas PW. Effects of dynactin disruption and dynein depletion on axonal microtubules. Traffic. 2006;7:524–537. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
