Glycoluril-tetrathiafulvalene molecular clips: on the influence of electronic and spatial properties for binding neutral accepting guests
- PMID: 26199657
- PMCID: PMC4505143
- DOI: 10.3762/bjoc.11.115
Glycoluril-tetrathiafulvalene molecular clips: on the influence of electronic and spatial properties for binding neutral accepting guests
Abstract
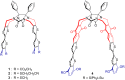
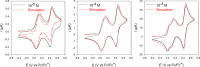
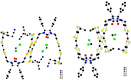
Glycoluril-based molecular clips incorporating tetrathiafulvalene (TTF) sidewalls have been synthesized through different strategies with the aim of investigating the effect of electrochemical and spatial properties for binding neutral accepting guests. We have in particular focused our study on the spacer extension in order to tune the intramolecular TTF···TTF distance within the clip and, consequently, the redox behavior of the receptor. Carried out at different concentrations in solution, electrochemical and spectroelectrochemical experiments provide evidence of mixed-valence and/or π-dimer intermolecular interactions between TTF units from two closed clips. The stepwise oxidation of each molecular clip involves an electrochemical mechanism with three one-electron processes and two charge-coupled chemical reactions, a scheme which is supported by electrochemical simulations. The fine-tunable π-donating ability of the TTF units and the cavity size allow to control binding interaction towards a strong electron acceptor such as tetrafluorotetracyanoquinodimethane (F4-TCNQ) or a weaker electron acceptor such as 1,3-dinitrobenzene (m-DNB).
Keywords: donor–acceptor interactions; glycoluril; molecular clips; supramolecular chemistry; tetrathiafulvalene.
Figures




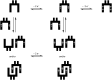






References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
