Advances in the synthesis of functionalised pyrrolotetrathiafulvalenes
- PMID: 26199667
- PMCID: PMC4505190
- DOI: 10.3762/bjoc.11.125
Advances in the synthesis of functionalised pyrrolotetrathiafulvalenes
Abstract
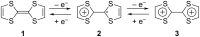
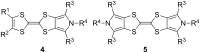
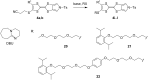
The electron-donor and unique redox properties of the tetrathiafulvalene (TTF, 1) moiety have led to diverse applications in many areas of chemistry. Monopyrrolotetrathiafulvalenes (MPTTFs, 4) and bispyrrolotetrathiafulvalenes (BPTTFs, 5) are useful structural motifs and have found widespread use in fields such as supramolecular chemistry and molecular electronics. Protocols enabling the synthesis of functionalised MPTTFs and BPTTFs are therefore of broad interest. Herein, we present the synthesis of a range of functionalised MPTTF and BPTTF species. Firstly, the large-scale preparation of the precursor species N-tosyl-(1,3)-dithiolo[4,5-c]pyrrole-2-one (6) is described, as well as the synthesis of the analogue N-tosyl-4,6-dimethyl-(1,3)-dithiolo[4,5-c]pyrrole-2-one (7). Thereafter, we show how 6 and 7 can be used to prepare BPTTFs using homocoupling reactions and functionalised MPTTFs using cross-coupling reactions with a variety of 1,3-dithiole-2-thiones (19). Subsequently, the incorporation of more complex functionality is discussed. We show how the 2-cyanoethyl protecting group can be used to afford MPTTFs functionalised with thioethers, exemplified by a series of ethylene glycol derivatives. Additionally, the merits of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) as an alternative to the most common deprotecting agent, CsOH·H2O are discussed. Finally, we show how a copper-mediated Ullman-type reaction can be applied to the N-arylation of MPTTFs and BPTTFs using a variety of aryl halides.
Keywords: Ullman coupling; heterocycles; protecting groups; sulfur chemistry; tetrathiafulvalene.
Figures

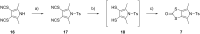
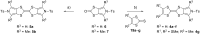


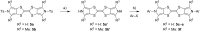
References
-
- Jeppesen J O, Becher J. Eur J Org Chem. 2003;2003:3245. doi: 10.1002/ejoc.200300078. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
