Efficacy and Safety of Orally Administered Intravenous Midazolam Versus a Commercially Prepared Syrup
- PMID: 26199710
- PMCID: PMC4505992
- DOI: 10.5812/ijp.25(3)2015.494
Efficacy and Safety of Orally Administered Intravenous Midazolam Versus a Commercially Prepared Syrup
Abstract
Background: Among different categories of sedative agents, benzodiazepines have been prescribed for more than three decades to patients of all ages. The effective and predictable sedative and amnestic effects of benzodiazepines support their use in pediatric patients. Midazolam is one of the most extensively used benzodiazepines in this age group. Oral form of drug is the best accepted route of administration in children.
Objectives: The purpose of this study was to compare the efficacy and safety of a commercially midazolam syrup versus orally administered IV midazolam in uncooperative dental patients. Second objective was to determine whether differences concerning sedation success can be explained by child's behavioral problems and dental fear.
Patients and methods: Eighty eight uncooperative dental patients (Frankl Scales 1,2) aged 3 to 6 years, and ASA I participated in this double blind, parallel randomized, controlled clinical trial. Midazolam was administered in a dose of 0.5 mg/kg for children under the age 5 and 0.2 mg/kg in patients over 5 years of age. Physiologic parameters including heart rate, respiratory rate, oxygen saturation and blood pressure were recorded. Behavior assessment was conducted throughout the course of treatment using Houpt Sedation Rating Scale and at critical moments of treatment (injection and cavity preparation) by North Carolina Scale. Dental fear and behavioral problems were evaluated using Child Fear Schedule Survey-Dental Subscale (CFSS-DS), and Strength and Difficulties Questionnaire (SDQ). Independent t-test, Chi-Square, and Pearson correlation were used for statistical analysis.
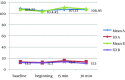
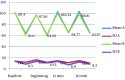
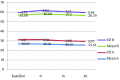
Results: Acceptable overall sedation ratings were observed in 90% and 86% of syrup and IV/Oral group respectively; Chi-Square P = 0.5. Other domains of Houpt Scale including: sleep, crying and movement were also not significantly different between groups. Physiological parameters remained in normal limits during study without significant difference between groups.
Conclusions: "Orally administered IV midazolam" preparation can be used as an alternative for commercially midazolam syrup.
Keywords: Child; Clinical Trial; Oral Midazolam; Sedation; Uncooperative Patient.
Figures
Similar articles
-
Two Oral Midazolam Preparations in Pediatric Dental Patients: A Prospective Randomised Clinical Trial.Int J Pediatr. 2015;2015:349795. doi: 10.1155/2015/349795. Epub 2015 May 20. Int J Pediatr. 2015. PMID: 26120325 Free PMC article.
-
Comparison of two Intranasal Sedatives, Midazolam versus Dexmedetomidine, in Children with High Dental Fear: a Randomized Clinical Trial.J Dent (Shiraz). 2022 Jun;23(2):129-136. doi: 10.30476/DENTJODS.2021.89323.1406. J Dent (Shiraz). 2022. PMID: 35783491 Free PMC article.
-
Parenting Styles and Sedation Efficacy in Pediatric Dental Care; A Study in Uncooperative Children Aged 4 to 6 Years: Structural Equation Modeling Approach.Med J Islam Repub Iran. 2024 Jul 30;38:87. doi: 10.47176/mjiri.38.87. eCollection 2024. Med J Islam Repub Iran. 2024. PMID: 39678768 Free PMC article.
-
Comparison of oral midazolam and triclofos in conscious sedation of uncooperative children.J Clin Pediatr Dent. 2011 Winter;36(2):189-96. doi: 10.17796/jcpd.36.2.0346178414pvw865. J Clin Pediatr Dent. 2011. PMID: 22524083 Clinical Trial.
-
Conscious Sedation Efficacy of 0.3 and 0.5 mg/kg Oral Midazolam for Three to Six Year-Old Uncooperative Children Undergoing Dental Treatment: A Clinical Trial.J Dent (Tehran). 2016 Mar;13(2):101-107. J Dent (Tehran). 2016. PMID: 27928238 Free PMC article.
Cited by
-
Comparison of nitrous oxide/midazolam and nitrous oxide/promethazine for pediatric dental sedation: A randomized, cross-over, clinical trial.Dent Res J (Isfahan). 2018 Nov-Dec;15(6):411-419. Dent Res J (Isfahan). 2018. PMID: 30534169 Free PMC article.
-
Efficacy of intranasal ketamine and midazolam for pediatric sedation: A double-blind, randomized clinical trial.Caspian J Intern Med. 2021 Fall;12(4):539-543. doi: 10.22088/cjim.12.4.539. Caspian J Intern Med. 2021. PMID: 34820060 Free PMC article.
-
Intranasal midazolam sedation as an effective sedation route in pediatric patients for radiologic imaging in the emergency ward: A single-blind randomized trial.Turk J Emerg Med. 2020 Oct 7;20(4):168-174. doi: 10.4103/2452-2473.297461. eCollection 2020 Oct-Dec. Turk J Emerg Med. 2020. PMID: 33089024 Free PMC article.
-
Single dose oral midazolam for minor emergency department procedures in children: a retrospective cohort study.J Pain Res. 2018 Feb 12;11:319-324. doi: 10.2147/JPR.S156080. eCollection 2018. J Pain Res. 2018. PMID: 29483782 Free PMC article.
References
-
- Raadal M, Lundeberg S, Haukali G. Pain, pain control and sedation. In: Koch G, Poulsen S, editors. Pediatric Dentistry, A Clinical Approach. 2 ed. Singapore: Blackwell; 2009. p. 54.
-
- Wilson S, Ganzberg L. Pediatric Dentistry, Infancy through Adolescence. 5 ed. China: Elsevier; 2013. Pain Reaction Control: Sedation. p. 98.
-
- McDonald R, Avery D. Dentistry for the child and adolescent. 9 ed. ,China: Mosby-Elsivier; 2013.
-
- Malamed S. Oral Sedation. 5 ed. California: Mosbey Elsevier; 2010. - DOI
-
- Reed MD, Rodarte A, Blumer JL, Khoo KC, Akbari B, Pou S, et al. The single-dose pharmacokinetics of midazolam and its primary metabolite in pediatric patients after oral and intravenous administration. J Clin Pharmacol. 2001;41(12):1359–69. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
