Route of antigen delivery impacts the immunostimulatory activity of dendritic cell-based vaccines for hepatocellular carcinoma
- PMID: 26199728
- PMCID: PMC4509479
- DOI: 10.1186/s40425-015-0077-x
Route of antigen delivery impacts the immunostimulatory activity of dendritic cell-based vaccines for hepatocellular carcinoma
Abstract
Background: Dendritic cells (DC) are uniquely equipped to capture, process, and present antigens from their environment. The context in which an antigen is acquired by DC helps to dictate the subsequent immune response. Cancer vaccination promotes antitumor immunity by directing an immune response to antigens expressed by tumors. We have tested the tumor-associated antigen alpha-fetoprotein (AFP) as an immunotherapy target. The majority of hepatocellular carcinomas (HCC) upregulate and secrete this oncofetal antigen.
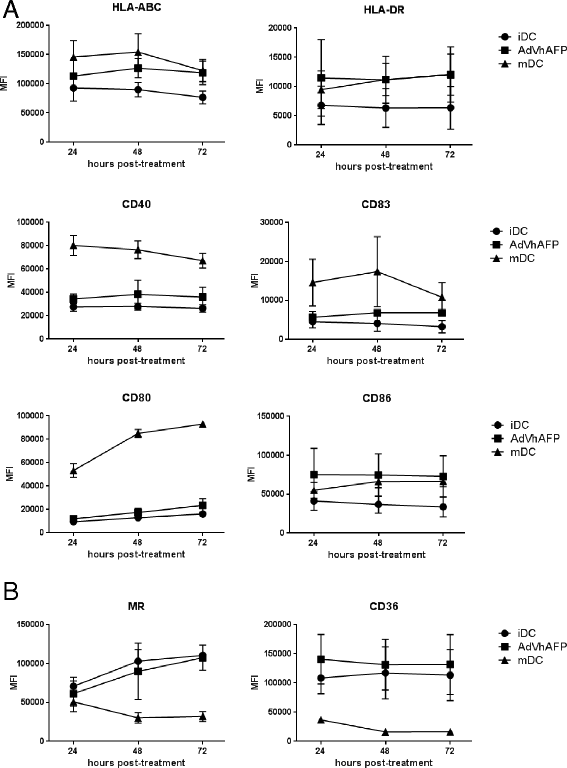
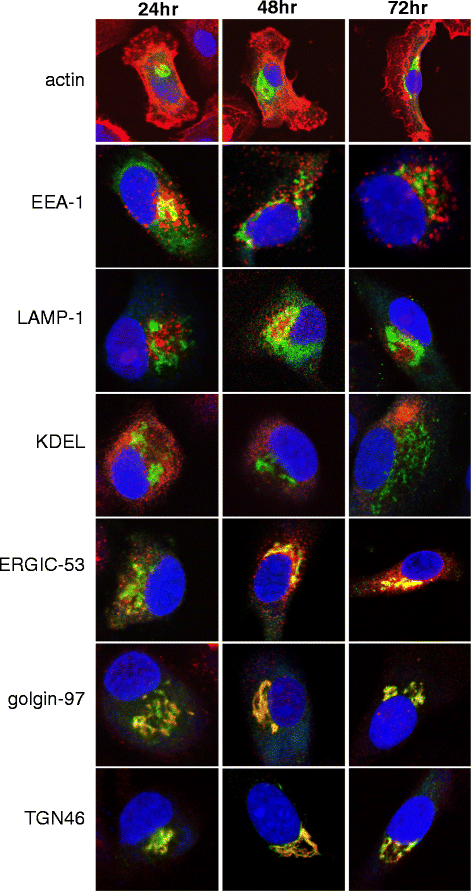
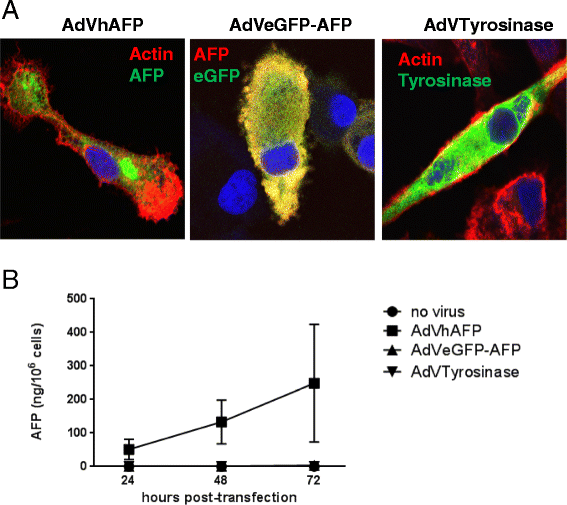
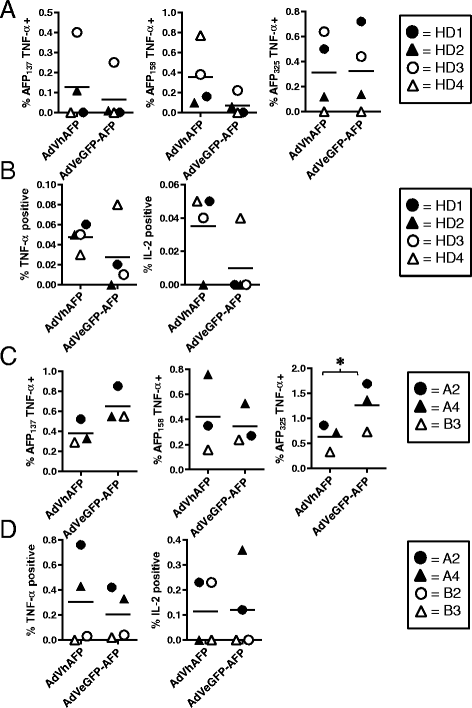
Methods: To develop cancer vaccines for HCC capable of promoting potent tumor-specific T cell responses, we tested adenovirally-encoded synthetic AFP, with or without its signal sequence, as well as protein forms of AFP and compared intracellular routing and subsequent antigen-specific CD8+ and CD4+ T cell responses.
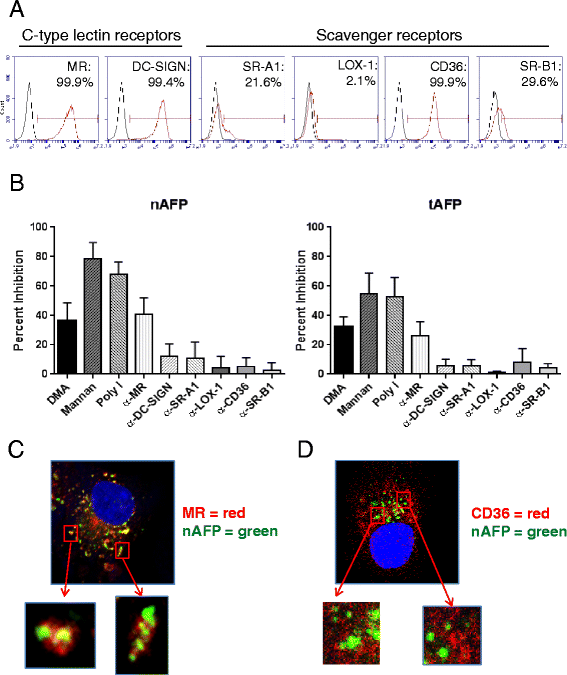
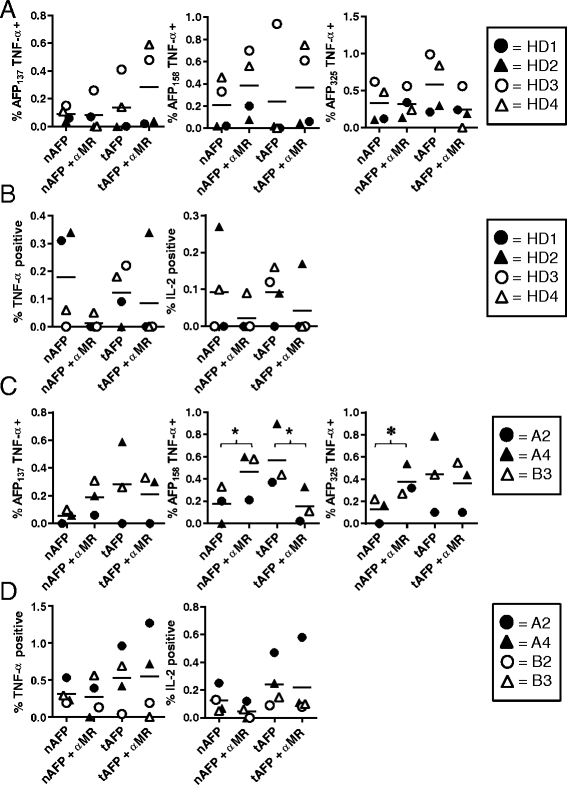
Results: Surprisingly, the secreted form of antigen was superior for both CD4+ and CD8+ T cell activation. We also examined the mechanism through which AFP protein is endocytosed and trafficked in human DC. We identify the mannose receptor (MR/CD206) as the primary uptake pathway for both normal cord blood-derived AFP (nAFP) and tumor-derived AFP (tAFP) proteins. While in healthy donors, nAFP and tAFP were cross-presented to CD8+ T cells similarly and CD4+ T cell responses were dependent upon MR-mediated uptake. In HCC patient cells, tAFP was more immunogenic, and CD4+ T cell responses were not MR-dependent.
Conclusions: Secreted, cytoplasmically retained, and endocytosed forms of AFP utilize unique uptake and processing pathways, resulting in different immunologic responses from the induced antigen-specific CD4+ and CD8+ T cells and between healthy donors and HCC patients. Collectively, these data elucidate pathways of spontaneous and induced anti-tumor immunity in HCC patients to this secreted antigen.
Keywords: Adenovirus; Alpha-fetoprotein; Dendritic cells; Hepatocellular carcinoma.
Figures

References
-
- Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182(2):389–400. doi: 10.1084/jem.182.2.389. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
