First-in-Human Evaluation of the Safety and Immunogenicity of a Recombinant Vesicular Stomatitis Virus Human Immunodeficiency Virus-1 gag Vaccine (HVTN 090)
- PMID: 26199949
- PMCID: PMC4504730
- DOI: 10.1093/ofid/ofv082
First-in-Human Evaluation of the Safety and Immunogenicity of a Recombinant Vesicular Stomatitis Virus Human Immunodeficiency Virus-1 gag Vaccine (HVTN 090)
Abstract
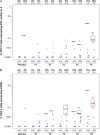
Background. We report the first-in-human safety and immunogenicity evaluation of a highly attenuated, replication-competent recombinant vesicular stomatitis virus (rVSV) human immunodeficiency virus (HIV)-1 vaccine. Methods. Sixty healthy, HIV-1-uninfected adults were enrolled in a randomized, double-blinded, placebo-controlled dose-escalation study. Groups of 12 participants received rVSV HIV-1 gag vaccine at 5 dose levels (4.6 × 10(3) to 3.4 × 10(7) particle forming units) (N = 10/group) or placebo (N = 2/group), delivered intramuscularly as bilateral injections at 0 and 2 months. Safety monitoring included VSV cultures from blood, urine, saliva, and swabs of oral lesions. Vesicular stomatitis virus-neutralizing antibodies, T-cell immunogenicity, and HIV-1 specific binding antibodies were assessed. Results. Local and systemic reactogenicity symptoms were mild to moderate and increased with dose. No severe reactogenicity or product-related serious adverse events were reported, and all rVSV cultures were negative. All vaccine recipients became seropositive for VSV after 2 vaccinations. gag-specific T-cell responses were detected in 63% of participants by interferon-γ enzyme-linked immunospot at the highest dose post boost. Conclusions. An attenuated replication-competent rVSV gag vaccine has an acceptable safety profile in healthy adults. This rVSV vector is a promising new vaccine platform for the development of vaccines to combat HIV-1 and other serious human diseases.
Keywords: HIV vaccine; dose-escalation; immunogenicity; safety; vesicular stomatitis virus.
Figures



References
-
- Baba TW, Liska V, Khimani AH et al. . Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med 1999; 5:194–203. Erratum in: Nat Med 1999 May; 5:590. - PubMed
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S et al. . Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361:2209–20. - PubMed
-
- Fields BN, Hawkins K. Human infection with the virus of vesicular stomatitis during an epizootic. N Engl J Med 1967; 277:989–94. - PubMed
-
- Reif JS, Webb PA, Monath TP et al. . Epizootic vesicular stomatitis in Colorado, 1982: infection in occupational risk groups. Am J Trop Med Hyg 1987; 36:177–82. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

