Interferon-λ: Immune Functions at Barrier Surfaces and Beyond
- PMID: 26200010
- PMCID: PMC4527169
- DOI: 10.1016/j.immuni.2015.07.001
Interferon-λ: Immune Functions at Barrier Surfaces and Beyond
Abstract
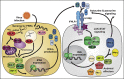
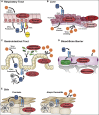
When type III interferon (IFN-λ; also known as interleukin-28 [IL-28] and IL-29) was discovered in 2003, its antiviral function was expected to be analogous to that of type I IFNs (IFN-α and IFN-β) via the induction of IFN-stimulated genes (ISGs). Although IFN-λ stimulates expression of antiviral ISGs preferentially in cells of epithelial origin, recent studies have defined additional antiviral mechanisms in other cell types and tissues. Viral infection models using mice lacking IFN-λ signaling and SNP associations with human disease have expanded our understanding of the contribution of IFN-λ to the antiviral response at anatomic barriers and the immune response beyond these barriers. In this review, we highlight recent insights into IFN-λ functions, including its ability to restrict virus spread into the brain and to clear chronic viral infections in the gastrointestinal tract. We also discuss how IFN-λ modulates innate and adaptive immunity, autoimmunity, and tumor progression and its possible therapeutic applications in human disease.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
References
-
- Angel J., Franco M.A., Greenberg H.B., Bass D. Lack of a role for type I and type II interferons in the resolution of rotavirus-induced diarrhea and infection in mice. J. Interferon Cytokine Res. 1999;19:655–659. - PubMed
-
- Ank N., Iversen M.B., Bartholdy C., Staeheli P., Hartmann R., Jensen U.B., Dagnaes-Hansen F., Thomsen A.R., Chen Z., Haugen H. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J. Immunol. 2008;180:2474–2485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

