The Glycogen Synthase Kinase 3α and β Isoforms Differentially Regulates Interleukin-12p40 Expression in Endothelial Cells Stimulated with Peptidoglycan from Staphylococcus aureus
- PMID: 26200352
- PMCID: PMC4511647
- DOI: 10.1371/journal.pone.0132867
The Glycogen Synthase Kinase 3α and β Isoforms Differentially Regulates Interleukin-12p40 Expression in Endothelial Cells Stimulated with Peptidoglycan from Staphylococcus aureus
Abstract
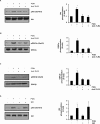
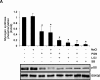
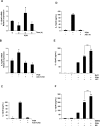
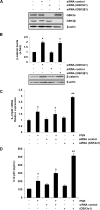
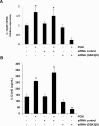
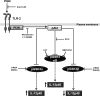
Glycogen synthase kinase 3 (GSK3) is a constitutively active regulatory enzyme that is important in cancer, diabetes, and cardiovascular, neurodegenerative, and psychiatric diseases. While GSK3α is usually important in neurodegenerative and psychiatric diseases GSK3β is fundamental in the inflammatory response caused by bacterial components. Peptidoglycan (PGN), one of the most abundant cell-wall structures of Gram-positive bacteria, is an important inducer of inflammation. To evaluate whether inhibition of GSK3α and GSK3β activity in bovine endothelial cells (BEC) regulates the expression of the pro-inflammatory cytokine IL-12p40, we treated BEC with SDS-purified PGN from Staphylococcus aureus. We found that PGN triggered a TLR2/PI3K/Akt-dependent phosphorylation of GSK3α at Ser21, GSK3β at Ser9, and NF-κB p65 subunit (p65) at Ser536, and the phosphorylation of GSK3α was consistently higher than that of GSK3β. The expression of IL-12p40 was inhibited in BEC stimulated with PGN and pre-treated with a specific neutralizing anti-TLR2 antibody that targets the extracellular domain of TLR2 or by the addition of Akt-i IV (an Akt inhibitor). Inhibition of GSK3α and GSK3β with LiCl or SB216763 induced an increase in IL-12p40 mRNA and protein. The effect of each isoform on IL-12p40 expression was evaluated by siRNA-gene expression silencing of GSK3α and GSK3β. GSK3α gene silencing resulted in a marked increase in IL-12p40 mRNA and protein while GSK3β gene silencing had the opposite effect on IL-12p40 expression. These results indicate that the TLR2/PI3K/Akt-dependent inhibition of GSK3α activity also plays an important role in the inflammatory response caused by stimulation of BEC with PGN from S. aureus.
Conflict of interest statement
Figures

Similar articles
-
Glycogen Synthase Kinase 3α Is the Main Isoform That Regulates the Transcription Factors Nuclear Factor-Kappa B and cAMP Response Element Binding in Bovine Endothelial Cells Infected with Staphylococcus aureus.Front Immunol. 2018 Jan 29;9:92. doi: 10.3389/fimmu.2018.00092. eCollection 2018. Front Immunol. 2018. PMID: 29434603 Free PMC article.
-
Glycogen synthase kinase 3 involvement in the excessive proinflammatory response to LPS in patients with decompensated cirrhosis.J Hepatol. 2011 Oct;55(4):784-93. doi: 10.1016/j.jhep.2010.12.039. Epub 2011 Feb 18. J Hepatol. 2011. PMID: 21334395
-
Peptidoglycan enhances proinflammatory cytokine expression through the TLR2 receptor, MyD88, phosphatidylinositol 3-kinase/AKT and NF-kappaB pathways in BV-2 microglia.Int Immunopharmacol. 2010 Aug;10(8):883-91. doi: 10.1016/j.intimp.2010.04.026. Epub 2010 May 5. Int Immunopharmacol. 2010. PMID: 20451669
-
Glycogen synthase kinase 3 (GSK3) in the heart: a point of integration in hypertrophic signalling and a therapeutic target? A critical analysis.Br J Pharmacol. 2008 Mar;153 Suppl 1(Suppl 1):S137-53. doi: 10.1038/sj.bjp.0707659. Epub 2008 Jan 21. Br J Pharmacol. 2008. PMID: 18204489 Free PMC article. Review.
-
GSK3 in Alzheimer's disease: mind the isoforms.J Alzheimers Dis. 2014;39(4):707-10. doi: 10.3233/JAD-131661. J Alzheimers Dis. 2014. PMID: 24254703 Free PMC article. Review.
Cited by
-
Targeting GSK3 and Associated Signaling Pathways Involved in Cancer.Cells. 2020 Apr 30;9(5):1110. doi: 10.3390/cells9051110. Cells. 2020. PMID: 32365809 Free PMC article. Review.
-
Glycogen Synthase Kinase 3β Modulates the Inflammatory Response Activated by Bacteria, Viruses, and Parasites.Front Immunol. 2021 May 4;12:675751. doi: 10.3389/fimmu.2021.675751. eCollection 2021. Front Immunol. 2021. PMID: 34017345 Free PMC article. Review.
-
Allostimulatory Effects of Dendritic Cells with Characteristic Features of a Regulatory Phenotype.PLoS One. 2016 Aug 15;11(8):e0159986. doi: 10.1371/journal.pone.0159986. eCollection 2016. PLoS One. 2016. PMID: 27525971 Free PMC article.
-
Research Progress on Signaling Pathway-Associated Oxidative Stress in Endothelial Cells.Oxid Med Cell Longev. 2017;2017:7156941. doi: 10.1155/2017/7156941. Epub 2017 Apr 19. Oxid Med Cell Longev. 2017. PMID: 28503253 Free PMC article. Review.
-
The role of PKA in the translational response to heat stress in Saccharomyces cerevisiae.PLoS One. 2017 Oct 18;12(10):e0185416. doi: 10.1371/journal.pone.0185416. eCollection 2017. PLoS One. 2017. PMID: 29045428 Free PMC article.
References
-
- McDonald C, Inohara N, Núñez G. Peptidoglycan signaling in innate immunity and inflammatory disease. J Biol Chem. 2005;280: 20177–20180. - PubMed
-
- Chen BC, Kang JC, Lu YT, Hsu MJ, Liao CC, Chiu WT, et al. Rac1 regulates peptidoglycan-induced nuclear factor-kappaB activation and cyclooxygenase-2 expression in RAW 264.7 macrophages by activating the phosphatidylinositol 3-kinase/Akt pathway. Mol Immunol. 2009;46: 1179–1188. 10.1016/j.molimm.2008.11.006 - DOI - PubMed
-
- Lin HY, Tang CH, Chen YH, Wei IH, Chen JH, Lai CH, et al. Peptidoglycan enhances proinflammatory cytokine expression through the TLR2 receptor, MyD88, phosphatidylinositol 3-kinase/AKT and NF-kappaB pathways in BV-2 microglia. Int Immunopharmacol. 2010;10: 883–891. 10.1016/j.intimp.2010.04.026 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

