From synthesis to function via iterative assembly of N-methyliminodiacetic acid boronate building blocks
- PMID: 26200460
- PMCID: PMC4688257
- DOI: 10.1021/acs.accounts.5b00128
From synthesis to function via iterative assembly of N-methyliminodiacetic acid boronate building blocks
Abstract
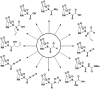
The study and optimization of small molecule function is often impeded by the time-intensive and specialist-dependent process that is typically used to make such compounds. In contrast, general and automated platforms have been developed for making peptides, oligonucleotides, and increasingly oligosaccharides, where synthesis is simplified to iterative applications of the same reactions. Inspired by the way natural products are biosynthesized via the iterative assembly of a defined set of building blocks, we developed a platform for small molecule synthesis involving the iterative coupling of haloboronic acids protected as the corresponding N-methyliminodiacetic acid (MIDA) boronates. Here we summarize our efforts thus far to develop this platform into a generalized and automated approach for small molecule synthesis. We and others have employed this approach to access many polyene-based compounds, including the polyene motifs found in >75% of all polyene natural products. This platform further allowed us to derivatize amphotericin B, the powerful and resistance-evasive but also highly toxic last line of defense in treating systemic fungal infections, and thereby understand its mechanism of action. This synthesis-enabled mechanistic understanding has led us to develop less toxic derivatives currently under evaluation as improved antifungal agents. To access more Csp(3)-containing small molecules, we gained a stereocontrolled entry into chiral, non-racemic α-boryl aldehydes through the discovery of a chiral derivative of MIDA. These α-boryl aldehydes are versatile intermediates for the synthesis of many Csp(3) boronate building blocks that are otherwise difficult to access. In addition, we demonstrated the utility of these types of building blocks in accessing pharmaceutically relevant targets via an iterative Csp(3) cross-coupling cycle. We have further expanded the scope of the platform to include stereochemically complex macrocyclic and polycyclic molecules using a linear-to-cyclized strategy, in which Csp(3) boronate building blocks are iteratively assembled into linear precursors that are then cyclized into the cyclic frameworks found in many natural products and natural product-like structures. Enabled by the serendipitous discovery of a catch-and-release protocol for generally purifying MIDA boronate intermediates, the platform has been automated. The synthesis of 14 distinct classes of small molecules, including pharmaceuticals, materials components, and polycyclic natural products, has been achieved using this new synthesis machine. It is anticipated that the scope of small molecules accessible by this platform will continue to expand via further developments in building block synthesis, Csp(3) cross-coupling methodologies, and cyclization strategies. Achieving these goals will enable the more generalized synthesis of small molecules and thereby help shift the rate-limiting step in small molecule science from synthesis to function.
Figures











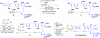
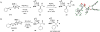

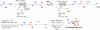
References
-
- Merrifield RB. Automated Synthesis of Peptides. Science. 1965;150:178–185. - PubMed
-
- Caruthers MH. Gene Synthesis Machines: DNA Chemistry and its Uses. Science. 1985;230:281–285. - PubMed
-
- Plante OJ, Palmacci ER, Seeberger PH. Automated Solid Phase Synthesis of Oligosaccharides. Science. 2001;291:1523–1527. - PubMed
-
- Dewick PM. Medicinal Natural Products: A Biosynthetic Approach. 3rd Wiley; 2009.
-
- Vitaku E, Smith ET, Njardarson JT. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014;57:10257–10274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

