Mid-Season Estimates of Influenza Vaccine Effectiveness against Influenza A(H3N2) Hospitalization in the Elderly in Quebec, Canada, January 2015
- PMID: 26200655
- PMCID: PMC4511737
- DOI: 10.1371/journal.pone.0132195
Mid-Season Estimates of Influenza Vaccine Effectiveness against Influenza A(H3N2) Hospitalization in the Elderly in Quebec, Canada, January 2015
Abstract
Background: The 2014/15 influenza season in Canada was characterized by an early epidemic due to vaccine-mismatched influenza A(H3N2) viruses, disproportionately affecting elderly individuals ≥65-years-old. We assessed vaccine effectiveness (VE) against A(H3N2) hospitalization among elderly individuals during the peak weeks of the 2014/15 epidemic in Quebec, Canada.
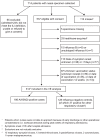
Methods: Nasal specimens and clinical/epidemiological data were collected within 7 days of illness onset from elderly patients admitted with respiratory symptoms to one of four participating hospitals between November 30, 2014 and January 13, 2015. Cases tested RT-PCR positive for influenza A(H3N2) and controls tested negative for any influenza. VE was assessed by test-negative case-control design.
Results: There were 314 participants including 186 cases (62% vaccinated) and 128 controls (59% vaccinated) included in primary VE analysis. Median age was 81.5 years, two-thirds were admitted from the community and 91% had underlying comorbidity. Crude VE against A(H3N2) hospitalization was -17% (95%CI: -86% to 26%), decreasing to -23% (95%CI: -99 to 23%) with adjustment for age and comorbidity, and to -39% (95%CI: -142 to 20%) with additional adjustment for specimen collection interval, calendar time, type of residence and hospital. In sensitivity analyses, VE estimates were improved toward the null with restriction to participants admitted from the community (-2%; 95%CI: -105 to 49%) or with specimen collection ≤4 days since illness onset (- 8%; 95%CI: -104 to 43%) but further from the null with restriction to participants with comorbidity (-51%; 95%CI: -169 to 15%).
Conclusion: The 2014/15 mismatched influenza vaccine provided elderly patients with no cross-protection against hospitalization with the A(H3N2) epidemic strain, reinforcing the need for adjunct protective measures among high-risk individuals and improved vaccine options.
Conflict of interest statement
Figures

References
-
- Public Health Agency of Canada. FluWatch: January 18 to January 24, 2015 (week 03). Public Health Agency of Canada, 2015. Available: http://www.phac-aspc.gc.ca/fluwatch/14-15/w03_15/pdf/fw2015-03-eng.pdf.
-
- Skowronski D, Chambers C, Sabaiduc S, De Serres G, Dickinson J, Winter A, et al. Interim estimates of 2014/15 vaccine effectiveness against influenza A(H3N2) from Canada's Sentinel Physician Surveillance Network, January 2015. Euro Surveill. 2015;20(4):1–18. - PubMed
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–40. - PubMed
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–86. - PubMed
-
- Skowronski DM, Janjua NZ, De Serres G, Sabaiduc S, Eshaghi A, Dickinson JA, et al. Low 2012–13 Influenza Vaccine Effectiveness Associated with Mutation in the Egg-Adapted H3N2 Vaccine Strain Not Antigenic Drift in Circulating Viruses. PloS One. 2014;9(3):e92153 10.1371/journal.pone.0092153 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

