DMSO induces dehydration near lipid membrane surfaces
- PMID: 26200868
- PMCID: PMC4621616
- DOI: 10.1016/j.bpj.2015.06.011
DMSO induces dehydration near lipid membrane surfaces
Abstract
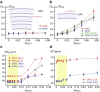
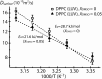
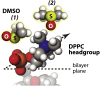
Dimethyl sulfoxide (DMSO) has been broadly used in biology as a cosolvent, a cryoprotectant, and an enhancer of membrane permeability, leading to the general assumption that DMSO-induced structural changes in cell membranes and their hydration water play important functional roles. Although the effects of DMSO on the membrane structure and the headgroup dehydration have been extensively studied, the mechanism by which DMSO invokes its effect on lipid membranes and the direct role of water in this process are unresolved. By directly probing the translational water diffusivity near unconfined lipid vesicle surfaces, the lipid headgroup mobility, and the repeat distances in multilamellar vesicles, we found that DMSO exclusively weakens the surface water network near the lipid membrane at a bulk DMSO mole fraction (XDMSO) of <0.1, regardless of the lipid composition and the lipid phase. Specifically, DMSO was found to effectively destabilize the hydration water structure at the lipid membrane surface at XDMSO <0.1, lower the energetic barrier to dehydrate this surface water, whose displacement otherwise requires a higher activation energy, consequently yielding compressed interbilayer distances in multilamellar vesicles at equilibrium with unaltered bilayer thicknesses. At XDMSO >0.1, DMSO enters the lipid interface and restricts the lipid headgroup motion. We postulate that DMSO acts as an efficient cryoprotectant even at low concentrations by exclusively disrupting the water network near the lipid membrane surface, weakening the cohesion between water and adhesion of water to the lipid headgroups, and so mitigating the stress induced by the volume change of water during freeze-thaw.
Copyright © 2015 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Correlating steric hydration forces with water dynamics through surface force and diffusion NMR measurements in a lipid-DMSO-H2O system.Proc Natl Acad Sci U S A. 2015 Aug 25;112(34):10708-13. doi: 10.1073/pnas.1512325112. Epub 2015 Aug 10. Proc Natl Acad Sci U S A. 2015. PMID: 26261313 Free PMC article.
-
Lipid membrane structure and interactions in dimethyl sulfoxide/water mixtures.Biophys J. 1998 Nov;75(5):2343-51. doi: 10.1016/S0006-3495(98)77678-7. Biophys J. 1998. PMID: 9788929 Free PMC article.
-
Communication: Contrasting effects of glycerol and DMSO on lipid membrane surface hydration dynamics and forces.J Chem Phys. 2016 Jul 28;145(4):041101. doi: 10.1063/1.4959904. J Chem Phys. 2016. PMID: 27475340 Free PMC article.
-
Dynamics and state of lipid bilayer-internal water unraveled with solution state 1H dynamic nuclear polarization.Phys Chem Chem Phys. 2011 May 7;13(17):7732-46. doi: 10.1039/c0cp02512g. Epub 2011 Mar 21. Phys Chem Chem Phys. 2011. PMID: 21423982
-
Controlled formation and mixing of two-dimensional fluids.Nano Lett. 2007 Jul;7(7):1980-4. doi: 10.1021/nl070726u. Epub 2007 Jun 6. Nano Lett. 2007. PMID: 17550298 Review.
Cited by
-
A single-molecule assessment of the protective effect of DMSO against DNA double-strand breaks induced by photo-and γ-ray-irradiation, and freezing.Sci Rep. 2017 Aug 17;7(1):8557. doi: 10.1038/s41598-017-08894-y. Sci Rep. 2017. PMID: 28819291 Free PMC article.
-
Vm-related extracellular potentials observed in red blood cells.Sci Rep. 2021 Sep 30;11(1):19446. doi: 10.1038/s41598-021-98102-9. Sci Rep. 2021. PMID: 34593849 Free PMC article.
-
Liposome Formulations for the Strategic Delivery of PARP1 Inhibitors: Development and Optimization.Nanomaterials (Basel). 2023 May 11;13(10):1613. doi: 10.3390/nano13101613. Nanomaterials (Basel). 2023. PMID: 37242030 Free PMC article.
-
Unveiling a Hidden Event in Fluorescence Correlative Microscopy by AFM Nanomechanical Analysis.Front Mol Biosci. 2021 May 6;8:669361. doi: 10.3389/fmolb.2021.669361. eCollection 2021. Front Mol Biosci. 2021. PMID: 34026842 Free PMC article.
-
Location of the TEMPO moiety of TEMPO-PC in phosphatidylcholine bilayers is membrane phase dependent.Biophys J. 2022 Jul 5;121(13):2550-2556. doi: 10.1016/j.bpj.2022.05.044. Epub 2022 May 31. Biophys J. 2022. PMID: 35651317 Free PMC article.
References
-
- Yu Z.W., Quinn P.J. Dimethyl sulphoxide: a review of its applications in cell biology. Biosci. Rep. 1994;14:259–281. - PubMed
-
- Anchordoguy T.J., Cecchini C.A., Crowe L.M. Insights into the cryoprotective mechanism of dimethyl sulfoxide for phospholipid bilayers. Cryobiology. 1991;28:467–473. - PubMed
-
- Rall W.F., Fahy G.M. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature. 1985;313:573–575. - PubMed
-
- Lovelock J.E., Bishop M.W.H. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature. 1959;183:1394–1395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

