Stabilizing the central part of tropomyosin increases the bending stiffness of the thin filament
- PMID: 26200873
- PMCID: PMC4621613
- DOI: 10.1016/j.bpj.2015.06.006
Stabilizing the central part of tropomyosin increases the bending stiffness of the thin filament
Abstract
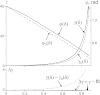
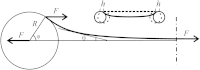
A two-beam optical trap was used to measure the bending stiffness of F-actin and reconstructed thin filaments. A dumbbell was formed by a filament segment attached to two beads that were held in the two optical traps. One trap was static and held a bead used as a force transducer, whereas an acoustooptical deflector moved the beam holding the second bead, causing stretch of the dumbbell. The distance between the beads was measured using image analysis of micrographs. An exact solution to the problem of bending of an elastic filament attached to two beads and subjected to a stretch was used for data analysis. Substitution of noncanonical residues in the central part of tropomyosin with canonical ones, G126R and D137L, and especially their combination, caused an increase in the bending stiffness of the thin filaments. The data confirm that the effect of these mutations on the regulation of actin-myosin interactions may be caused by an increase in tropomyosin stiffness.
Copyright © 2015 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Cooperativity of myosin interaction with thin filaments is enhanced by stabilizing substitutions in tropomyosin.J Muscle Res Cell Motil. 2017 Apr;38(2):183-191. doi: 10.1007/s10974-017-9472-x. Epub 2017 May 24. J Muscle Res Cell Motil. 2017. PMID: 28540577
-
Functional role of the core gap in the middle part of tropomyosin.FEBS J. 2018 Mar;285(5):871-886. doi: 10.1111/febs.14369. Epub 2018 Jan 16. FEBS J. 2018. PMID: 29278453
-
Effect of Cardiomyopathic Mutations in Tropomyosin on Calcium Regulation of the Actin-Myosin Interaction in Skeletal Muscle.Bull Exp Biol Med. 2016 Nov;162(1):42-44. doi: 10.1007/s10517-016-3540-x. Epub 2016 Nov 23. Bull Exp Biol Med. 2016. PMID: 27878731
-
Structure of the F-actin-tropomyosin complex.Nature. 2015 Mar 5;519(7541):114-7. doi: 10.1038/nature14033. Epub 2014 Dec 1. Nature. 2015. PMID: 25470062 Free PMC article.
-
Structural basis for bending tropomyosin around actin in muscle thin filaments.Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8165-6. doi: 10.1073/pnas.151265198. Proc Natl Acad Sci U S A. 2001. PMID: 11459946 Free PMC article. Review. No abstract available.
Cited by
-
Structural and Functional Peculiarities of Cytoplasmic Tropomyosin Isoforms, the Products of TPM1 and TPM4 Genes.Int J Mol Sci. 2021 May 13;22(10):5141. doi: 10.3390/ijms22105141. Int J Mol Sci. 2021. PMID: 34067970 Free PMC article.
-
Functional and Structural Properties of Cytoplasmic Tropomyosin Isoforms Tpm1.8 and Tpm1.9.Int J Mol Sci. 2024 Jun 22;25(13):6873. doi: 10.3390/ijms25136873. Int J Mol Sci. 2024. PMID: 38999987 Free PMC article.
-
Optical Tweezers Approaches for Probing Multiscale Protein Mechanics and Assembly.Front Mol Biosci. 2020 Oct 6;7:577314. doi: 10.3389/fmolb.2020.577314. eCollection 2020. Front Mol Biosci. 2020. PMID: 33134316 Free PMC article. Review.
-
Functional outcomes of structural peculiarities of striated muscle tropomyosin.J Muscle Res Cell Motil. 2020 Mar;41(1):55-70. doi: 10.1007/s10974-019-09552-8. Epub 2019 Sep 18. J Muscle Res Cell Motil. 2020. PMID: 31535252 Review.
-
The Relaxation Properties of Myofibrils Are Compromised by Amino Acids that Stabilize α-Tropomyosin.Biophys J. 2017 Jan 24;112(2):376-387. doi: 10.1016/j.bpj.2016.12.013. Biophys J. 2017. PMID: 28122223 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

