A human ventricular myocyte model with a refined representation of excitation-contraction coupling
- PMID: 26200878
- PMCID: PMC4621846
- DOI: 10.1016/j.bpj.2015.06.017
A human ventricular myocyte model with a refined representation of excitation-contraction coupling
Abstract
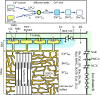
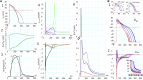
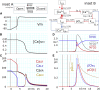
Cardiac Ca(2+)-induced Ca(2+) release (CICR) occurs by a regenerative activation of ryanodine receptors (RyRs) within each Ca(2+)-releasing unit, triggered by the activation of L-type Ca(2+) channels (LCCs). CICR is then terminated, most probably by depletion of Ca(2+) in the junctional sarcoplasmic reticulum (SR). Hinch et al. previously developed a tightly coupled LCC-RyR mathematical model, known as the Hinch model, that enables simulations to deal with a variety of functional states of whole-cell populations of a Ca(2+)-releasing unit using a personal computer. In this study, we developed a membrane excitation-contraction model of the human ventricular myocyte, which we call the human ventricular cell (HuVEC) model. This model is a hybrid of the most recent HuVEC models and the Hinch model. We modified the Hinch model to reproduce the regenerative activation and termination of CICR. In particular, we removed the inactivated RyR state and separated the single step of RyR activation by LCCs into triggering and regenerative steps. More importantly, we included the experimental measurement of a transient rise in Ca(2+) concentrations ([Ca(2+)], 10-15 μM) during CICR in the vicinity of Ca(2+)-releasing sites, and thereby calculated the effects of the local Ca(2+) gradient on CICR as well as membrane excitation. This HuVEC model successfully reconstructed both membrane excitation and key properties of CICR. The time course of CICR evoked by an action potential was accounted for by autonomous changes in an instantaneous equilibrium open probability of couplons. This autonomous time course was driven by a core feedback loop including the pivotal local [Ca(2+)], influenced by a time-dependent decay in the SR Ca(2+) content during CICR.
Copyright © 2015 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
A novel mechanism of tandem activation of ryanodine receptors by cytosolic and SR luminal Ca2+ during excitation-contraction coupling in atrial myocytes.J Physiol. 2017 Jun 15;595(12):3835-3845. doi: 10.1113/JP273611. Epub 2017 Feb 1. J Physiol. 2017. PMID: 28028837 Free PMC article.
-
An integrative model of the cardiac ventricular myocyte incorporating local control of Ca2+ release.Biophys J. 2002 Dec;83(6):2918-45. doi: 10.1016/S0006-3495(02)75301-0. Biophys J. 2002. PMID: 12496068 Free PMC article.
-
Mechanisms of excitation-contraction coupling in an integrative model of the cardiac ventricular myocyte.Biophys J. 2006 Jan 1;90(1):77-91. doi: 10.1529/biophysj.105.065169. Epub 2005 Oct 7. Biophys J. 2006. PMID: 16214852 Free PMC article.
-
Local control in cardiac E-C coupling.J Mol Cell Cardiol. 2012 Feb;52(2):298-303. doi: 10.1016/j.yjmcc.2011.04.014. Epub 2011 May 14. J Mol Cell Cardiol. 2012. PMID: 21586292 Review.
-
Epac in cardiac calcium signaling.J Mol Cell Cardiol. 2013 May;58:162-71. doi: 10.1016/j.yjmcc.2012.11.021. Epub 2012 Dec 7. J Mol Cell Cardiol. 2013. PMID: 23220153 Review.
Cited by
-
Study of the union method of microelectrode array and AFM for the recording of electromechanical activities in living cardiomyocytes.Eur Biophys J. 2017 Jul;46(5):495-507. doi: 10.1007/s00249-016-1192-4. Epub 2016 Dec 23. Eur Biophys J. 2017. PMID: 28012038
-
Novel Two-Step Classifier for Torsades de Pointes Risk Stratification from Direct Features.Front Pharmacol. 2017 Nov 14;8:816. doi: 10.3389/fphar.2017.00816. eCollection 2017. Front Pharmacol. 2017. PMID: 29184497 Free PMC article.
-
Bifurcations and Proarrhythmic Behaviors in Cardiac Electrical Excitations.Biomolecules. 2022 Mar 16;12(3):459. doi: 10.3390/biom12030459. Biomolecules. 2022. PMID: 35327651 Free PMC article. Review.
-
Mechanisms Underlying Spontaneous Action Potential Generation Induced by Catecholamine in Pulmonary Vein Cardiomyocytes: A Simulation Study.Int J Mol Sci. 2019 Jun 14;20(12):2913. doi: 10.3390/ijms20122913. Int J Mol Sci. 2019. PMID: 31207916 Free PMC article.
-
External K+ dependence of strong inward rectifier K+ channel conductance is caused not by K+ but by competitive pore blockade by external Na.J Gen Physiol. 2018 Jul 2;150(7):977-989. doi: 10.1085/jgp.201711936. Epub 2018 Jun 15. J Gen Physiol. 2018. PMID: 29907600 Free PMC article.
References
-
- Priebe L., Beuckelmann D.J. Simulation study of cellular electric properties in heart failure. Circ. Res. 1998;82:1206–1223. - PubMed
-
- Ten Tusscher K.H., Bernus O., Panfilov A.V. Comparison of electrophysiological models for human ventricular cells and tissues. Prog. Biophys. Mol. Biol. 2006;90:326–345. - PubMed
-
- ten Tusscher K.H., Noble D., Panfilov A.V. A model for human ventricular tissue. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1573–H1589. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

