The DNA-Binding Domain of S. pombe Mrc1 (Claspin) Acts to Enhance Stalling at Replication Barriers
- PMID: 26201080
- PMCID: PMC4511789
- DOI: 10.1371/journal.pone.0132595
The DNA-Binding Domain of S. pombe Mrc1 (Claspin) Acts to Enhance Stalling at Replication Barriers
Abstract
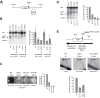
During S-phase replication forks can stall at specific genetic loci. At some loci, the stalling events depend on the replisome components Schizosaccharomyces pombe Swi1 (Saccharomyces cerevisiae Tof1) and Swi3 (S. cerevisiae Csm3) as well as factors that bind DNA in a site-specific manner. Using a new genetic screen we identified Mrc1 (S. cerevisiae Mrc1/metazoan Claspin) as a replisome component involved in replication stalling. Mrc1 is known to form a sub-complex with Swi1 and Swi3 within the replisome and is required for the intra-S phase checkpoint activation. This discovery is surprising as several studies show that S. cerevisiae Mrc1 is not required for replication barrier activity. In contrast, we show that deletion of S. pombe mrc1 leads to an approximately three-fold reduction in barrier activity at several barriers and that Mrc1's role in replication fork stalling is independent of its role in checkpoint activation. Instead, S. pombe Mrc1 mediated fork stalling requires the presence of a functional copy of its phylogenetically conserved DNA binding domain. Interestingly, this domain is on the sequence level absent from S. cerevisiae Mrc1. Our study indicates that direct interactions between the eukaryotic replisome and the DNA are important for site-specific replication stalling.
Conflict of interest statement
Figures






Similar articles
-
Fission yeast Swi1-Swi3 complex facilitates DNA binding of Mrc1.J Biol Chem. 2010 Dec 17;285(51):39609-22. doi: 10.1074/jbc.M110.173344. Epub 2010 Oct 5. J Biol Chem. 2010. PMID: 20924116 Free PMC article.
-
Interactions between Swi1-Swi3, Mrc1 and S phase kinase, Hsk1 may regulate cellular responses to stalled replication forks in fission yeast.Genes Cells. 2009 Jun;14(6):669-82. doi: 10.1111/j.1365-2443.2009.01300.x. Epub 2009 Apr 30. Genes Cells. 2009. PMID: 19422421 Free PMC article.
-
Schizosaccharomyces pombe Swi1, Swi3, and Hsk1 are components of a novel S-phase response pathway to alkylation damage.Mol Cell Biol. 2005 Apr;25(7):2770-84. doi: 10.1128/MCB.25.7.2770-2784.2005. Mol Cell Biol. 2005. PMID: 15767681 Free PMC article.
-
DNA replication: stalling a fork for imprinting and switching.Curr Biol. 2004 Nov 9;14(21):R915-7. doi: 10.1016/j.cub.2004.10.012. Curr Biol. 2004. PMID: 15530381 Review.
-
Mrc1/Claspin: a new role for regulation of origin firing.Curr Genet. 2017 Oct;63(5):813-818. doi: 10.1007/s00294-017-0690-y. Epub 2017 Mar 29. Curr Genet. 2017. PMID: 28357499 Review.
Cited by
-
New insights into donor directionality of mating-type switching in Schizosaccharomyces pombe.PLoS Genet. 2018 May 31;14(5):e1007424. doi: 10.1371/journal.pgen.1007424. eCollection 2018 May. PLoS Genet. 2018. PMID: 29852001 Free PMC article.
-
Differences in microorganism profile in periprosthetic joint infections of the hip in patients affected by chronic kidney disease.J Orthop Traumatol. 2024 Dec 19;25(1):67. doi: 10.1186/s10195-024-00806-x. J Orthop Traumatol. 2024. PMID: 39702802 Free PMC article.
-
The fork protection complex promotes parental histone recycling and epigenetic memory.Cell. 2024 Sep 5;187(18):5029-5047.e21. doi: 10.1016/j.cell.2024.07.017. Epub 2024 Aug 1. Cell. 2024. PMID: 39094569 Free PMC article.
-
The Fission Yeast Mating-Type Switching Motto: "One-for-Two" and "Two-for-One".Microbiol Mol Biol Rev. 2023 Mar 21;87(1):e0000821. doi: 10.1128/mmbr.00008-21. Epub 2023 Jan 11. Microbiol Mol Biol Rev. 2023. PMID: 36629411 Free PMC article. Review.
-
The Balancing Act of Ribonucleotides in DNA.Trends Biochem Sci. 2016 May;41(5):434-445. doi: 10.1016/j.tibs.2016.02.005. Epub 2016 Mar 17. Trends Biochem Sci. 2016. PMID: 26996833 Free PMC article. Review.
References
-
- Dalgaard JZ, Godfrey EL, MacFarlane RJ (2011) Eukaryotic Replication Barriers: How. Why and Where Forks Stall In: Seligmann H, editor. DNA Replication-Current Advances: INTECH; pp. 269–304.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

