Single-cell RNA-seq transcriptome analysis of linear and circular RNAs in mouse preimplantation embryos
- PMID: 26201400
- PMCID: PMC4511241
- DOI: 10.1186/s13059-015-0706-1
Single-cell RNA-seq transcriptome analysis of linear and circular RNAs in mouse preimplantation embryos
Abstract
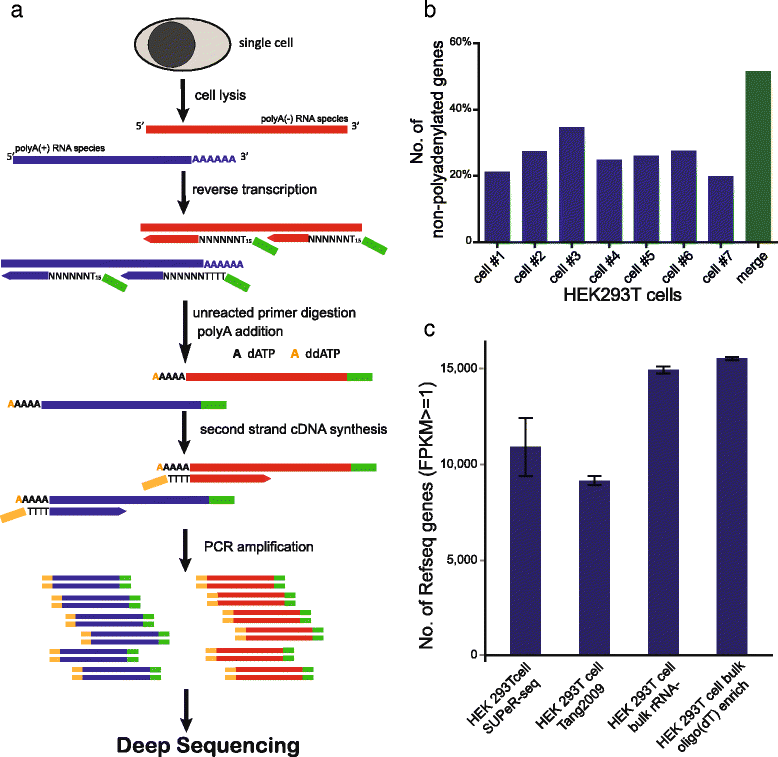
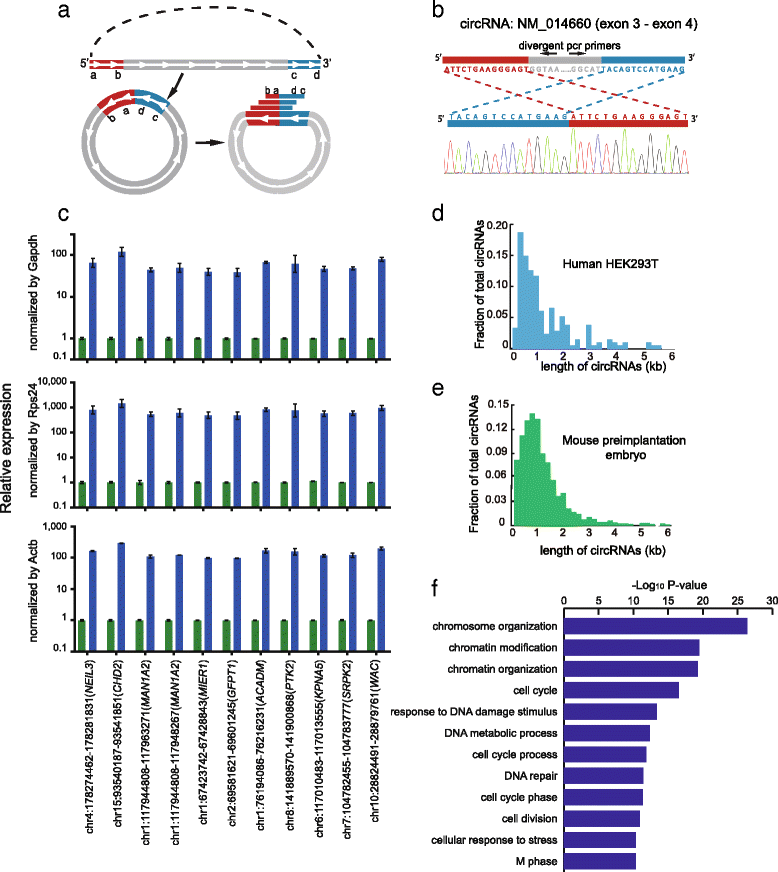
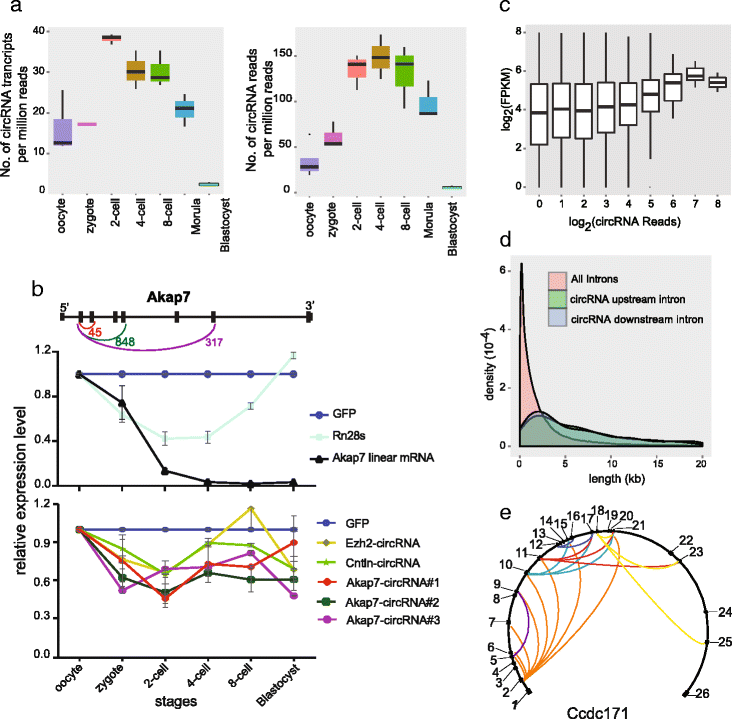
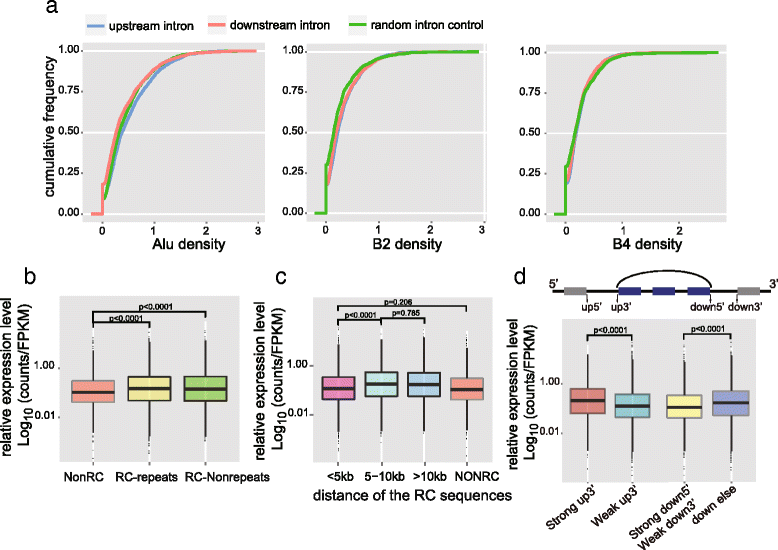
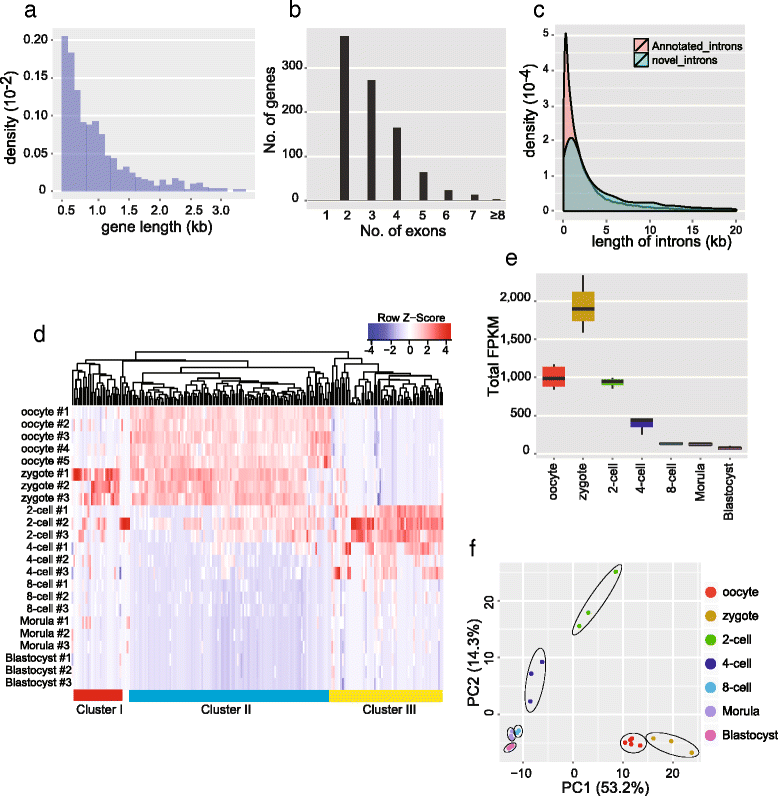
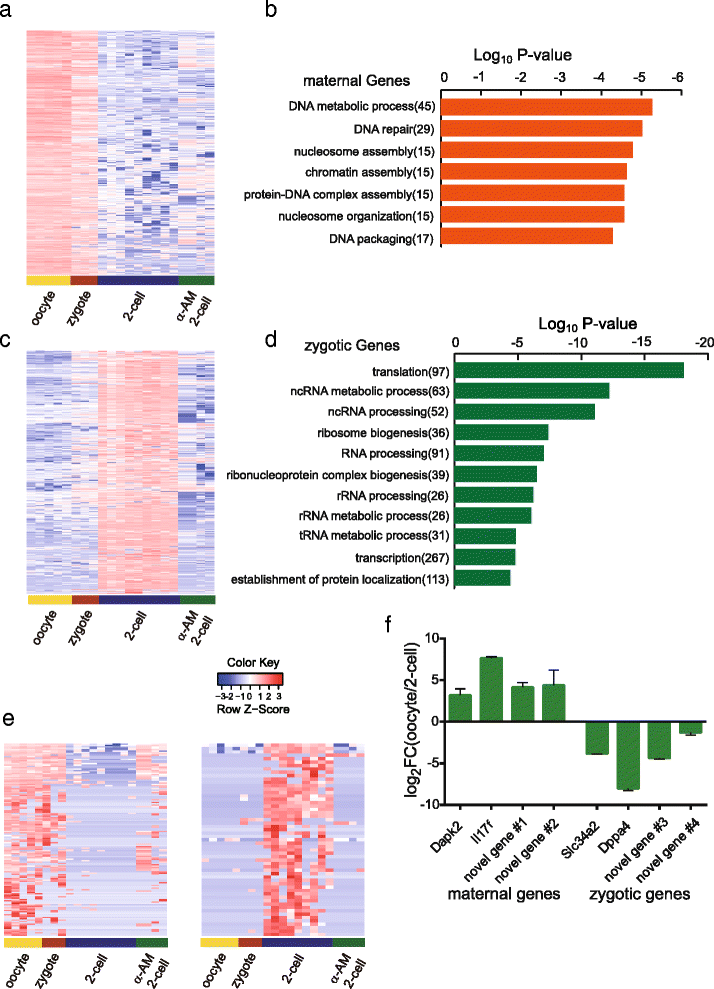
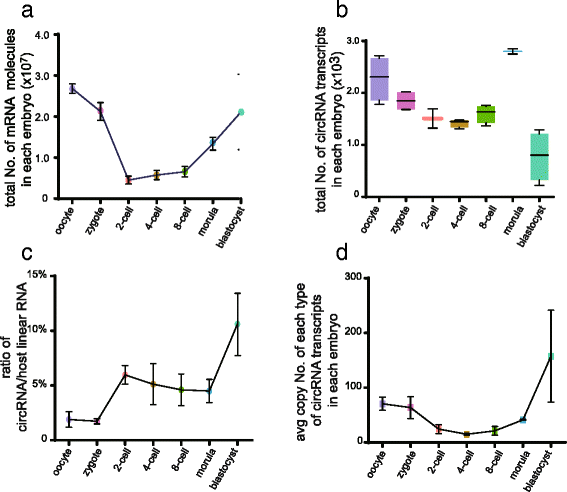
Circular RNAs (circRNAs) are a new class of non-polyadenylated non-coding RNAs that may play important roles in many biological processes. Here we develop a single-cell universal poly(A)-independent RNA sequencing (SUPeR-seq) method to sequence both polyadenylated and non-polyadenylated RNAs from individual cells. This method exhibits robust sensitivity, precision and accuracy. We discover 2891 circRNAs and 913 novel linear transcripts in mouse preimplantation embryos and further analyze the abundance of circRNAs along development, the function of enriched genes, and sequence features of circRNAs. Our work is key to deciphering regulation mechanisms of circRNAs during mammalian early embryonic development.
Figures
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

