D-amino acid inhibits biofilm but not new bone formation in an ovine model
- PMID: 26201421
- PMCID: PMC4626484
- DOI: 10.1007/s11999-015-4465-9
D-amino acid inhibits biofilm but not new bone formation in an ovine model
Abstract
Background: Infectious complications of musculoskeletal trauma are an important factor contributing to patient morbidity. Biofilm-dispersive bone grafts augmented with D-amino acids (D-AAs) prevent biofilm formation in vitro and in vivo, but the effects of D-AAs on osteocompatibility and new bone formation have not been investigated.
Questions/purposes: We asked: (1) Do D-AAs hinder osteoblast and osteoclast differentiation in vitro? (2) Does local delivery of D-AAs from low-viscosity bone grafts inhibit new bone formation in a large-animal model?
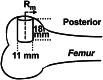
Methods: Methicillin-sensitive Staphylococcus aureus and methicillin-resistant S aureus clinical isolates, mouse bone marrow stromal cells, and osteoclast precursor cells were treated with an equal mass (1:1:1) mixture of D-Pro:D-Met:D-Phe. The effects of the D-AA dose on biofilm inhibition (n = 4), biofilm dispersion (n = 4), and bone marrow stromal cell proliferation (n = 3) were quantitatively measured by crystal violet staining. Osteoblast differentiation was quantitatively assessed by alkaline phosphatase staining, von Kossa staining, and quantitative reverse transcription for the osteogenic factors a1Col1 and Ocn (n = 3). Osteoclast differentiation was quantitatively measured by tartrate-resistant acid phosphatase staining (n = 3). Bone grafts augmented with 0 or 200 mmol/L D-AAs were injected in ovine femoral condyle defects in four sheep. New bone formation was evaluated by μCT and histology 4 months later. An a priori power analysis indicated that a sample size of four would detect a 7.5% difference of bone volume/total volume between groups assuming a mean and SD of 30% and 5%, respectively, with a power of 80% and an alpha level of 0.05 using a two-tailed t-test between the means of two independent samples.
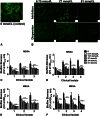
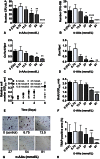
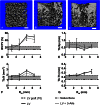
Results: Bone marrow stromal cell proliferation, osteoblast differentiation, and osteoclast differentiation were inhibited at D-AAs concentrations of 27 mmol/L or greater in a dose-responsive manner in vitro (p < 0.05). In methicillin-sensitive and methicillin-resistant S aureus clinical isolates, D-AAs inhibited biofilm formation at concentrations of 13.5 mmol/L or greater in vitro (p < 0.05). Local delivery of D-AAs from low-viscosity grafts did not inhibit new bone formation in a large-animal model pilot study (0 mmol/L D-AAs: bone volume/total volume = 26.9% ± 4.1%; 200 mmol/L D-AAs: bone volume/total volume = 28.3% ± 15.4%; mean difference with 95% CI = -1.4; p = 0.13).
Conclusions: D-AAs inhibit biofilm formation, bone marrow stromal cell proliferation, osteoblast differentiation, and osteoclast differentiation in vitro in a dose-responsive manner. Local delivery of D-AAs from bone grafts did not inhibit new bone formation in vivo at clinically relevant doses.
Clinical relevance: Local delivery of D-AAs is an effective antibiofilm strategy that does not appear to inhibit bone repair. Longitudinal studies investigating bacterial burden, bone formation, and bone remodeling in contaminated defects as a function of D-AA dose are required to further support the use of D-AAs in the clinical management of infected open fractures.
Figures

Comment in
-
CORR Insights(®): D-amino acid inhibits biofilm but not new bone formation in an ovine model.Clin Orthop Relat Res. 2015 Dec;473(12):3962-4. doi: 10.1007/s11999-015-4517-1. Epub 2015 Aug 27. Clin Orthop Relat Res. 2015. PMID: 26310678 Free PMC article. No abstract available.
Similar articles
-
CORR Insights(®): D-amino acid inhibits biofilm but not new bone formation in an ovine model.Clin Orthop Relat Res. 2015 Dec;473(12):3962-4. doi: 10.1007/s11999-015-4517-1. Epub 2015 Aug 27. Clin Orthop Relat Res. 2015. PMID: 26310678 Free PMC article. No abstract available.
-
Effects of local delivery of D-amino acids from biofilm-dispersive scaffolds on infection in contaminated rat segmental defects.Biomaterials. 2013 Oct;34(30):7533-43. doi: 10.1016/j.biomaterials.2013.06.026. Epub 2013 Jul 5. Biomaterials. 2013. PMID: 23831189
-
Staphylococcus aureus biofilms decrease osteoblast viability, inhibits osteogenic differentiation, and increases bone resorption in vitro.BMC Musculoskelet Disord. 2013 Jun 14;14:187. doi: 10.1186/1471-2474-14-187. BMC Musculoskelet Disord. 2013. PMID: 23767824 Free PMC article.
-
Cellular and molecular effects of growth hormone and estrogen on human bone cells.APMIS Suppl. 1997;71:1-30. APMIS Suppl. 1997. PMID: 9357492 Review.
-
Effect of vancomycin-coated tympanostomy tubes on methicillin-resistant Staphylococcus aureus biofilm formation: in vitro study.J Laryngol Otol. 2010 Jun;124(6):594-8. doi: 10.1017/S0022215109992672. Epub 2010 Jan 8. J Laryngol Otol. 2010. PMID: 20056010 Review.
Cited by
-
Lysostaphin and BMP-2 co-delivery reduces S. aureus infection and regenerates critical-sized segmental bone defects.Sci Adv. 2019 May 17;5(5):eaaw1228. doi: 10.1126/sciadv.aaw1228. eCollection 2019 May. Sci Adv. 2019. PMID: 31114804 Free PMC article.
-
Eradication of Enterococcus faecalis Biofilms on Human Dentin.Front Microbiol. 2016 Dec 26;7:2055. doi: 10.3389/fmicb.2016.02055. eCollection 2016. Front Microbiol. 2016. PMID: 28082955 Free PMC article.
-
Approaches to Dispersing Medical Biofilms.Microorganisms. 2017 Apr 1;5(2):15. doi: 10.3390/microorganisms5020015. Microorganisms. 2017. PMID: 28368320 Free PMC article. Review.
-
Multifaceted Enhancement of L-Leucine-Enriched Ovine Bone Graft: Physicochemical Characteristics and Osteogenic Potential for Improved Guided Bone Regeneration.Cureus. 2024 Jul 12;16(7):e64416. doi: 10.7759/cureus.64416. eCollection 2024 Jul. Cureus. 2024. PMID: 39131038 Free PMC article.
-
Methicillin-resistant Staphylococcus aureus (MRSA): antibiotic-resistance and the biofilm phenotype.Medchemcomm. 2019 Mar 14;10(8):1231-1241. doi: 10.1039/c9md00044e. eCollection 2019 Aug 1. Medchemcomm. 2019. PMID: 31534648 Free PMC article. Review.
References
-
- Bindl R, Oheim R, Pogoda P, Beil FT, Gruchenberg K, Reitmaier S, Wehner T, Calcia E, Radermacher P, Claes L, Amling M, Ignatius A. Metaphyseal fracture healing in a sheep model of low turnover osteoporosis induced by hypothalamic-pituitary disconnection (HPD) J Orthop Res. 2013;31:1851–1857. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

