Non-sedation versus sedation with a daily wake-up trial in critically ill patients receiving mechanical ventilation--effects on physical function: study protocol for a randomized controlled trial: a substudy of the NONSEDA trial
- PMID: 26201718
- PMCID: PMC4511451
- DOI: 10.1186/s13063-015-0856-1
Non-sedation versus sedation with a daily wake-up trial in critically ill patients receiving mechanical ventilation--effects on physical function: study protocol for a randomized controlled trial: a substudy of the NONSEDA trial
Abstract
Background: Critically ill patients rapidly loose much of their muscle mass and strength. This can be attributed to prolonged admission, prolonged mechanical ventilation and increased mortality, and it can have a negative impact on the degree of independence and quality of life. In the NONSEDA trial we randomize critically ill patients to non-sedation or sedation with a daily wake-up trial during mechanical ventilation in the intensive care unit. It has never been assessed whether non-sedation affects physical function. The aim of this study is to assess the effects of non-sedation versus sedation with a daily wake-up trial on physical function after discharge from intensive care unit.
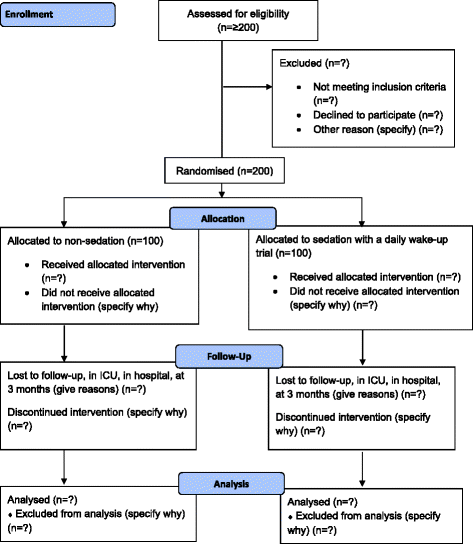
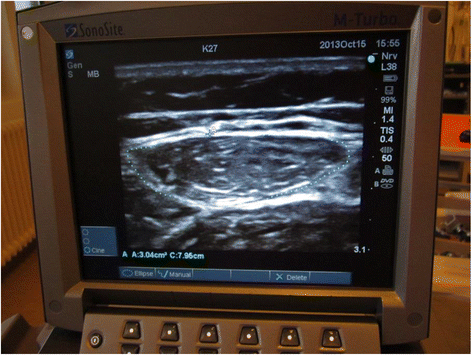
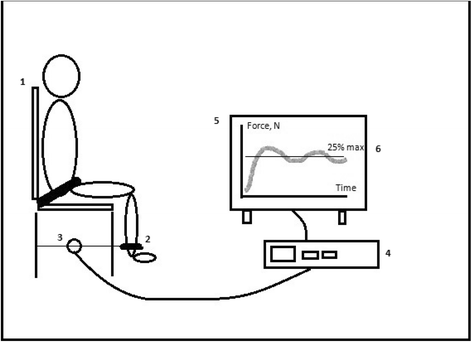
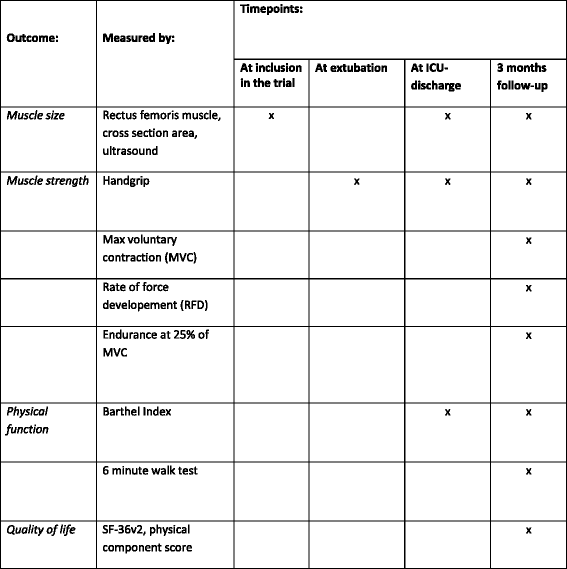
Methods/design: Investigator-initiated, randomized, clinical, parallel-group, superiority trial, including 700 patients in total, with a substudy concerning 200 of these patients. Inclusion criteria will be intubated, mechanically ventilated patients with expected duration of mechanical ventilation >24 h. Exclusion criteria will be patients with severe head trauma, coma at admission or status epilepticus, patients treated with therapeutic hypothermia, patients with PaO2/FiO2<9 where sedation might be necessary to ensure sufficient oxygenation or placing the patient in a prone position. The experimental intervention will be non-sedation supplemented with pain management during mechanical ventilation. The control intervention will be sedation with a daily wake-up trial. The co-primary outcome will be quality of life regarding physical function (SF-36, physical component) and degree of independence in activities of daily living (Barthel Index), and this will be assessed for all 700 patients participating in the NONSEDA trial. The secondary outcomes, which will be assessed for the subpopulation of 200 NONSEDA patients in the trial site, Kolding, will be 6-min walking distance, handgrip strength, muscle size (ultrasonographic measurement of the rectus femoris muscle cross-sectional area) and biomechanical data on lower extremity function (maximal voluntary contraction, rate of force development and endurance).
Discussion: This study is the first to investigate the effect of no sedation during critical illness on physical function. If an effect is found, it will add important information on how to prevent muscle weakness following critical illness.
Trial registration: The study has been approved by the relevant scientific ethics committee and is registered at ClinicalTrials.gov (ID: NCT02034942, 9 January 2014).
Figures
References
-
- Danish Intensive Care Database, DID. [in Danish] Available at: https://www.sundhed.dk/content/cms/12/4712_aarsrapport-2011-did-290612.pdf. Accessed September 10, 2013.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

