Enzymatic hydroxylation of an unactivated methylene C-H bond guided by molecular dynamics simulations
- PMID: 26201742
- PMCID: PMC4518477
- DOI: 10.1038/nchem.2285
Enzymatic hydroxylation of an unactivated methylene C-H bond guided by molecular dynamics simulations
Abstract
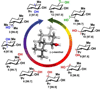
The hallmark of enzymes from secondary metabolic pathways is the pairing of powerful reactivity with exquisite site selectivity. The application of these biocatalytic tools in organic synthesis, however, remains under-utilized due to limitations in substrate scope and scalability. Here, we report how the reactivity of a monooxygenase (PikC) from the pikromycin pathway is modified through computationally guided protein and substrate engineering, and applied to the oxidation of unactivated methylene C-H bonds. Molecular dynamics and quantum mechanical calculations were used to develop a predictive model for substrate scope, site selectivity and stereoselectivity of PikC-mediated C-H oxidation. A suite of menthol derivatives was screened computationally and evaluated through in vitro reactions, where each substrate adhered to the predicted models for selectivity and conversion to product. This platform was also expanded beyond menthol-based substrates to the selective hydroxylation of a variety of substrate cores ranging from cyclic to fused bicyclic and bridged bicyclic compounds.
Figures
Similar articles
-
Molecular Dynamics and QM/MM Calculations Predict the Substrate-Induced Gating of Cytochrome P450 BM3 and the Regio- and Stereoselectivity of Fatty Acid Hydroxylation.J Am Chem Soc. 2016 Jan 27;138(3):837-45. doi: 10.1021/jacs.5b08737. Epub 2016 Jan 13. J Am Chem Soc. 2016. PMID: 26716578
-
Regio- and stereoselectivity in the CYP450BM3-catalyzed hydroxylation of complex terpenoids: a QM/MM study.Phys Chem Chem Phys. 2020 Oct 7;22(38):21696-21706. doi: 10.1039/d0cp03083j. Phys Chem Chem Phys. 2020. PMID: 32969450
-
Selective oxidation of carbolide C-H bonds by an engineered macrolide P450 mono-oxygenase.Proc Natl Acad Sci U S A. 2009 Nov 3;106(44):18463-8. doi: 10.1073/pnas.0907203106. Epub 2009 Oct 15. Proc Natl Acad Sci U S A. 2009. PMID: 19833867 Free PMC article.
-
Differential behavior of the sub-sites of cytochrome 450 active site in binding of substrates, and products (implications for coupling/uncoupling).Biochim Biophys Acta. 2007 Mar;1770(3):360-75. doi: 10.1016/j.bbagen.2006.09.018. Epub 2006 Oct 5. Biochim Biophys Acta. 2007. PMID: 17134838 Review.
-
Use of chemical auxiliaries to control p450 enzymes for predictable oxidations at unactivated C-h bonds of substrates.Adv Exp Med Biol. 2015;851:209-28. doi: 10.1007/978-3-319-16009-2_8. Adv Exp Med Biol. 2015. PMID: 26002737 Free PMC article. Review.
Cited by
-
Discovery, design, and engineering of enzymes based on molecular retrobiosynthesis.mLife. 2025 Mar 28;4(2):107-125. doi: 10.1002/mlf2.70009. eCollection 2025 Apr. mLife. 2025. PMID: 40313979 Free PMC article. Review.
-
Molecular Dynamics Simulations Guide Chimeragenesis and Engineered Control of Chemoselectivity in Diketopiperazine Dimerases.Angew Chem Int Ed Engl. 2023 May 8;62(20):e202210254. doi: 10.1002/anie.202210254. Epub 2023 Feb 17. Angew Chem Int Ed Engl. 2023. PMID: 36610039 Free PMC article.
-
Understanding and Improving the Activity of Flavin-Dependent Halogenases via Random and Targeted Mutagenesis.Annu Rev Biochem. 2018 Jun 20;87:159-185. doi: 10.1146/annurev-biochem-062917-012042. Epub 2018 Mar 28. Annu Rev Biochem. 2018. PMID: 29589959 Free PMC article. Review.
-
Harnessing natural product assembly lines: structure, promiscuity, and engineering.J Ind Microbiol Biotechnol. 2016 Mar;43(2-3):371-87. doi: 10.1007/s10295-015-1704-8. Epub 2015 Nov 2. J Ind Microbiol Biotechnol. 2016. PMID: 26527577 Free PMC article. Review.
-
Highly Enantioselective Oxidation of Nonactivated Aliphatic C-H Bonds with Hydrogen Peroxide Catalyzed by Manganese Complexes.ACS Cent Sci. 2017 Mar 22;3(3):196-204. doi: 10.1021/acscentsci.6b00368. Epub 2017 Feb 8. ACS Cent Sci. 2017. PMID: 28386597 Free PMC article.
References
-
- Gormisky PE, White MC. Catalyst-controlled aliphatic C-H oxidations with a predictive model for site-selectivity. J.. Am. Chem. Soc. 2013;135:14052–14055. - PubMed
-
- Chen MS, White MC. Combined effects on selectivity in Fe-catalyzed methylene oxidation. Science. 2010;327:566–571. - PubMed
-
- Roiban GD, Agudo R, Reetz MT. Cytochrome P450 catalyzed oxidative hydroxylation of achiral organic compounds with simultaneous creation of two chirality centers in a single C-H activation step. Angew. Chem. Int. Ed. 2014;53:8659–8663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

