Randomized, placebo-controlled trial of incobotulinumtoxina for upper-limb post-stroke spasticity
- PMID: 26201835
- PMCID: PMC5064747
- DOI: 10.1002/mus.24776
Randomized, placebo-controlled trial of incobotulinumtoxina for upper-limb post-stroke spasticity
Erratum in
-
Erratum.Muscle Nerve. 2016 Jun;54(1):170. doi: 10.1002/mus.25182. Muscle Nerve. 2016. PMID: 27297960 Free PMC article. No abstract available.
Abstract
Introduction: Efficacy and safety of incobotulinumtoxinA in post-stroke upper-limb spasticity were studied.
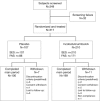
Methods: Subjects randomized 2:1 to incobotulinumtoxinA (fixed dose 400 U) or placebo, with fixed doses for the primary target clinical pattern (PTCP; flexed elbow, 200 U; flexed wrist, 150 U; clenched fist, 100 U). Doses for non-primary patterns were flexible within predefined ranges.
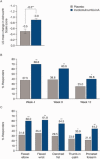
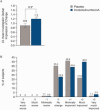
Results: At week 4, incobotulinumtoxinA led to larger improvements in PTCP Ashworth scale (AS) scores than placebo [least-squares mean change ± standard error: -0.9 ± 0.06 (n = 171) vs. -0.5 ± 0.08 (n = 88); P < 0.001], and more subjects were PTCP AS responders (≥1-point improvement) with incobotulinumtoxinA (69.6%) than with placebo (37.5%; P < 0.001). Investigator's Global Impression of Change confirmed superiority of incobotulinumtoxinA vs. placebo (P = 0.003). IncobotulinumtoxinA was associated with functional improvements, as demonstrated in responder rates for Disability Assessment Scale principal target at week 4 (P = 0.007). Adverse events were mainly mild/moderate, and were reported by 22.4% (incobotulinumtoxinA) and 16.8% (placebo) of subjects.
Conclusions: IncobotulinumtoxinA significantly improved upper-limb spasticity and associated disability, and was well-tolerated.
Keywords: Xeomin; botulinum toxins, type A; incobotulinumtoxinA; muscle spasticity; stroke.
© 2015 The Authors. Muscle and Nerve published by Wiley Periodicals, Inc.
Figures
Similar articles
-
IncobotulinumtoxinA: A Review in Upper Limb Spasticity.Drugs. 2016 Sep;76(14):1373-9. doi: 10.1007/s40265-016-0630-z. Drugs. 2016. PMID: 27530616 Review.
-
IncobotulinumtoxinA Efficacy and Safety in Adults with Upper-Limb Spasticity Following Stroke: Results from the Open-Label Extension Period of a Phase 3 Study.Adv Ther. 2019 Jan;36(1):187-199. doi: 10.1007/s12325-018-0833-7. Epub 2018 Nov 27. Adv Ther. 2019. PMID: 30484117 Free PMC article. Clinical Trial.
-
Efficacy and safety of incobotulinumtoxinA in post-stroke upper-limb spasticity in Japanese subjects: results from a randomized, double-blind, placebo-controlled study (J-PURE).J Neurol. 2020 Jul;267(7):2029-2041. doi: 10.1007/s00415-020-09777-5. Epub 2020 Mar 26. J Neurol. 2020. PMID: 32219557 Free PMC article. Clinical Trial.
-
Sustained efficacy of incobotulinumtoxina repeated injections for upper-limb post-stroke spasticity: A post hoc analysis.J Rehabil Med. 2021 Jan 5;53(1):jrm00138. doi: 10.2340/16501977-2760. J Rehabil Med. 2021. PMID: 33112408 Free PMC article. Clinical Trial.
-
Botulinum Toxin Type A for Upper Limb Spasticity in Poststroke Patients: A Meta-analysis of Randomized Controlled Trials.J Stroke Cerebrovasc Dis. 2020 Jun;29(6):104682. doi: 10.1016/j.jstrokecerebrovasdis.2020.104682. Epub 2020 Apr 15. J Stroke Cerebrovasc Dis. 2020. PMID: 32305277 Review.
Cited by
-
Clinical neurophysiology in the treatment of movement disorders: IFCN handbook chapter.Clin Neurophysiol. 2024 Aug;164:57-99. doi: 10.1016/j.clinph.2024.05.007. Epub 2024 May 23. Clin Neurophysiol. 2024. PMID: 38852434 Free PMC article. Review.
-
IncobotulinumtoxinA: A Review in Upper Limb Spasticity.Drugs. 2016 Sep;76(14):1373-9. doi: 10.1007/s40265-016-0630-z. Drugs. 2016. PMID: 27530616 Review.
-
Use of botulinum toxin for movement disorders.Drugs Context. 2019 Jun 18;8:212586. doi: 10.7573/dic.212586. eCollection 2019. Drugs Context. 2019. PMID: 31258617 Free PMC article. Review.
-
Safety and Efficacy of HU-014 in the Treatment of Post-Stroke Upper Limb Spasticity: A Phase I Pilot Study.Toxins (Basel). 2022 Oct 25;14(11):730. doi: 10.3390/toxins14110730. Toxins (Basel). 2022. PMID: 36355980 Free PMC article. Clinical Trial.
-
IncobotulinumtoxinA for the treatment of lower-limb spasticity in children and adolescents with cerebral palsy: A phase 3 study.J Pediatr Rehabil Med. 2021;14(2):183-197. doi: 10.3233/PRM-210040. J Pediatr Rehabil Med. 2021. PMID: 34092664 Free PMC article. Clinical Trial.
References
-
- Kanovský P, Slawek J, Denes Z, Platz T, Sassin I, Comes G, et al Efficacy and safety of botulinum neurotoxin NT 201 in poststroke upper‐limb spasticity. Clin Neuropharmacol 2009;32:259–265. - PubMed
-
- Bakheit AM, Thilmann AF, Ward AB, Poewe W, Wissel J, Muller J, et al A randomized, double‐blind, placebo‐controlled, dose‐ranging study to compare the efficacy and safety of three doses of botulinum toxin type A (Dysport) with placebo in upper‐limb spasticity after stroke. Stroke 2000;31:2402–2406. - PubMed
-
- Brashear A, Gordon MF, Elovic E, Kassicieh VD, Marciniak C, Do M, et al Intramuscular injection of botulinum toxin for the treatment of wrist and finger spasticity after a stroke. N Engl J Med 2002;347:395–400. - PubMed
-
- Simpson DM, Alexander DN, O'Brien CF, Tagliati M, Aswad AS, Leon JM, et al Botulinum toxin type A in the treatment of upper extremity spasticity: a randomized, double‐blind, placebo‐controlled trial. Neurology 1996;46:1306–1310. - PubMed
-
- Simpson DM, Gracies JM, Yablon SA, Barbano R, Brashear A, BoNT/TZD Study Team . Botulinum neurotoxin versus tizanidine in upper‐limb spasticity: a placebo‐controlled study. J Neurol Neurosurg Psychiatry 2009;80:380–385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

