Observational methods to assess the effectiveness of screening colonoscopy in reducing right colon cancer mortality risk: SCOLAR
- PMID: 26201973
- PMCID: PMC4666780
- DOI: 10.2217/cer.15.39
Observational methods to assess the effectiveness of screening colonoscopy in reducing right colon cancer mortality risk: SCOLAR
Abstract
Aims: Screening colonoscopy's effectiveness in reducing risk of death from right colon cancers remains unclear. Methodological challenges of existing observational studies addressing this issue motivated the design of 'Effectiveness of Screening for Colorectal Cancer in Average-Risk Adults (SCOLAR)'.
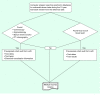
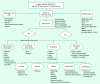
Methods: SCOLAR is a nested case-control study based on two large integrated health systems. This affords access to a large, well-defined historical cohort linked to integrated data on cancer outcomes, patient eligibility, test indications and important confounders.
Results: We found electronic data adequate for excluding ineligible patients (except family history), but not the detailed information needed for test indication assignment.
Conclusion: The lessons of SCOLAR's design and implementation may be useful for future studies seeking to evaluate the effectiveness of screening tests in community settings.
Keywords: cancer screening; case–control study; colorectal cancer; selection bias.
Conflict of interest statement
The study was supported by a grant from the National Cancer Institute at the US NIH (#U01 CA151736). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures
References
-
- Fedewa SA, Goodman M, Flanders WD, et al. Elimination of cost-sharing and receipt of screening for colorectal and breast cancer. Cancer. 2015 doi: 10.1002/cncr.29494. Epub ahead of print. - PubMed
-
- Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota colon cancer control study. N. Engl. J. Med. 1993;328(19):1365–1371. - PubMed
-
• Clinical trial demonstrating efficacy of guaiac fecal occult blood test in reducing risk of colorectal cancer (CRC) deaths.
-
- Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472–1477. - PubMed
-
- Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726):1624–1633. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
