Superbugs on Duodenoscopes: the Challenge of Cleaning and Disinfection of Reusable Devices
- PMID: 26202125
- PMCID: PMC4572565
- DOI: 10.1128/JCM.01394-15
Superbugs on Duodenoscopes: the Challenge of Cleaning and Disinfection of Reusable Devices
Abstract
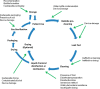
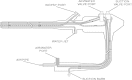
Inadequate flexible endoscope reprocessing has been associated with infection outbreaks, most recently caused by carbapenem-resistant Enterobacteriaceae. Lapses in essential device reprocessing steps such as cleaning, disinfection/sterilization, and storage have been reported, but some outbreaks have occurred despite claimed adherence to established guidelines. Recommended changes in these guidelines include the use of sterilization instead of high-level disinfection or the use of routine microbial culturing to monitor efficacy of reprocessing. This review describes the current standards for endoscope reprocessing, associated outbreaks, and the complexities associated with both microbiological culture and sterilization approaches to mitigating the risk of infection associated with endoscopy.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- McDonnell G, Sheard D. 2012. A practical guide to decontamination in healthcare. Wiley-Blackwell, Oxford, United Kingdom.
-
- ANSI/AAMI. 2013. ANSI/AAMI ST58: Chemical sterilization and high-level disinfection in health care facilities. Association for the Advancement of Medical Instrumentation, Arlington, VA.
-
- ANSI/AAMI. 2015. ST91: Flexible and semi-rigid endoscope processing in healthcare facilities. Association for the Advancement of Medical Instrumentation, Arlington, VA.
-
- ASGE Standards of Practice Committee, Banerjee S, Shen B, Nelson DB, Lichtenstein DR, Baron TH, Anderson MA, Dominitz JA, Gan SI, Harrison ME, Ikenberry SO, Jagannath SB, Fanelli RD, Lee K, van Guilder T, Stewart LE. 2008. Infection control during GI endoscopy. Gastrointest Endosc 67:781–790. doi:10.1016/j.gie.2008.01.027. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

