UTP activates small-conductance Ca2+-activated K+ channels in murine detrusor PDGFRα+ cells
- PMID: 26202222
- PMCID: PMC4572394
- DOI: 10.1152/ajprenal.00156.2015
UTP activates small-conductance Ca2+-activated K+ channels in murine detrusor PDGFRα+ cells
Abstract
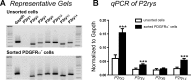
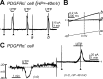
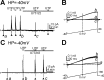
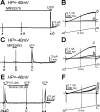
Purines induce transient contraction and prolonged relaxation of detrusor muscles. Transient contraction is likely due to activation of inward currents in smooth muscle cells, and prolonged relaxation may be due to activation of small-conductance Ca(2+)-activated K(+) (SK) channels via P2Y1 receptors expressed by detrusor PDGF receptor (PDGFR)α(+) cells. We investigated whether other subtypes of P2Y receptors are involved in the activation of SK channels in PDGFRα(+) cells of detrusor muscles. Quantitative analysis of transcripts revealed that P2ry2, P2ry4, and P2ry14 are expressed in PDGFRα(+) cells of P2ry1-deficient/enhanced green fluorescent protein (P2ry1(-/-)/eGFP) mice at similar levels as in wild-type mice. UTP, a P2Y2/P2Y4 agonist, activated large outward currents in detrusor PDGFRα(+) cells. SK channel blockers and an inhibitor of phospholipase C completely abolished currents activated by UTP. In contrast, UTP activated nonselective cation currents in smooth muscle cells. Under current-clamp (current = 0), UTP induced significant hyperpolarization of PDGFRα(+) cells. MRS2500, a selective P2Y1 antagonist, did not affect UTP-activated outward currents in PDGFRα(+) cells from wild-type mice, and activation of outward currents by UTP was retained in P2ry1(-/-)/eGFP mice. As a negative control, we tested the effect of MRS2693, a selective P2Y6 agonist. This compound did not activate outward currents in PDGFRα(+) cells, and currents activated by UTP were unaffected by MRS2578, a selective P2Y6 antagonist. The nonselective P2Y receptor blocker suramin inhibited UTP-activated outward currents in PDGFRα(+) cells. Our data demonstrate that P2Y2 and/or P2Y4 receptors function, in addition to P2Y1 receptors, in activating SK currents in PDGFRα(+) cells and possibly in mediating purinergic relaxation responses in detrusor muscles.
Keywords: detrusor relaxation; interstitial cells; potassium channels; purinergic receptors.
Copyright © 2015 the American Physiological Society.
Figures



Similar articles
-
Purinergic inhibitory regulation of murine detrusor muscles mediated by PDGFRα+ interstitial cells.J Physiol. 2014 Mar 15;592(6):1283-93. doi: 10.1113/jphysiol.2013.267989. Epub 2014 Jan 6. J Physiol. 2014. PMID: 24396055 Free PMC article.
-
Platelet-derived growth factor receptor-α-positive cells and not smooth muscle cells mediate purinergic hyperpolarization in murine colonic muscles.Am J Physiol Cell Physiol. 2014 Sep 15;307(6):C561-70. doi: 10.1152/ajpcell.00080.2014. Epub 2014 Jul 23. Am J Physiol Cell Physiol. 2014. PMID: 25055825 Free PMC article.
-
Functional expression of SK channels in murine detrusor PDGFR+ cells.J Physiol. 2013 Jan 15;591(2):503-13. doi: 10.1113/jphysiol.2012.241505. Epub 2012 Nov 12. J Physiol. 2013. PMID: 23148317 Free PMC article.
-
Molecular pharmacology of P2Y-receptors.Naunyn Schmiedebergs Arch Pharmacol. 2000 Nov;362(4-5):310-23. doi: 10.1007/s002100000310. Naunyn Schmiedebergs Arch Pharmacol. 2000. PMID: 11111826 Review.
-
How selective antagonists and genetic modification have helped characterise the expression and functions of vascular P2Y receptors.Purinergic Signal. 2025 Feb;21(1):11-22. doi: 10.1007/s11302-024-10016-z. Epub 2024 May 13. Purinergic Signal. 2025. PMID: 38740733 Free PMC article. Review.
Cited by
-
Octodon degus, a new model to study the agonist and plexus-induced response in the urinary bladder.J Physiol Biochem. 2017 Feb;73(1):77-87. doi: 10.1007/s13105-016-0527-z. Epub 2016 Oct 13. J Physiol Biochem. 2017. PMID: 27738973
-
Purinergic signalling in the urinary bladder - When function becomes dysfunction.Auton Neurosci. 2021 Nov;235:102852. doi: 10.1016/j.autneu.2021.102852. Epub 2021 Jul 17. Auton Neurosci. 2021. PMID: 34329833 Free PMC article.
-
Premature contractions of the bladder are suppressed by interactions between TRPV4 and SK3 channels in murine detrusor PDGFRα+ cells.Sci Rep. 2017 Sep 25;7(1):12245. doi: 10.1038/s41598-017-12561-7. Sci Rep. 2017. PMID: 28947806 Free PMC article.
-
Studies of ultrastructure, gene expression, and marker analysis reveal that mouse bladder PDGFRA+ interstitial cells are fibroblasts.Am J Physiol Renal Physiol. 2022 Sep 1;323(3):F299-F321. doi: 10.1152/ajprenal.00135.2022. Epub 2022 Jul 14. Am J Physiol Renal Physiol. 2022. PMID: 35834272 Free PMC article.
-
The Urothelium: Life in a Liquid Environment.Physiol Rev. 2020 Oct 1;100(4):1621-1705. doi: 10.1152/physrev.00041.2019. Epub 2020 Mar 19. Physiol Rev. 2020. PMID: 32191559 Free PMC article. Review.
References
-
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006. - PMC - PubMed
-
- Aronsson P, Andersson M, Ericsson T, Giglio D. Assessment and characterization of purinergic contractions and relaxations in the rat urinary bladder. Basic Clin Pharmacol Toxicol 107: 603–613, 2010. - PubMed
-
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572: 65–68, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

