Brain Invasion by Mouse Hepatitis Virus Depends on Impairment of Tight Junctions and Beta Interferon Production in Brain Microvascular Endothelial Cells
- PMID: 26202229
- PMCID: PMC4577898
- DOI: 10.1128/JVI.01501-15
Brain Invasion by Mouse Hepatitis Virus Depends on Impairment of Tight Junctions and Beta Interferon Production in Brain Microvascular Endothelial Cells
Abstract
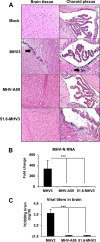
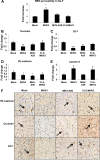
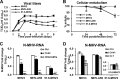
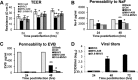
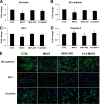
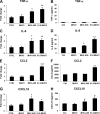
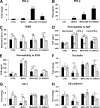
Coronaviruses (CoVs) have shown neuroinvasive properties in humans and animals secondary to replication in peripheral organs, but the mechanism of neuroinvasion is unknown. The major aim of our work was to evaluate the ability of CoVs to enter the central nervous system (CNS) through the blood-brain barrier (BBB). Using the highly hepatotropic mouse hepatitis virus type 3 (MHV3), its attenuated variant, 51.6-MHV3, which shows low tropism for endothelial cells, and the weakly hepatotropic MHV-A59 strain from the murine coronavirus group, we investigated the virus-induced dysfunctions of BBB in vivo and in brain microvascular endothelial cells (BMECs) in vitro. We report here a MHV strain-specific ability to cross the BBB during acute infection according to their virulence for liver. Brain invasion was observed only in MHV3-infected mice and correlated with enhanced BBB permeability associated with decreased expression of zona occludens protein 1 (ZO-1), VE-cadherin, and occludin, but not claudin-5, in the brain or in cultured BMECs. BBB breakdown in MHV3 infection was not related to production of barrier-dysregulating inflammatory cytokines or chemokines by infected BMECs but rather to a downregulation of barrier protective beta interferon (IFN-β) production. Our findings highlight the importance of IFN-β production by infected BMECs in preserving BBB function and preventing access of blood-borne infectious viruses to the brain.
Importance: Coronaviruses (CoVs) infect several mammals, including humans, and are associated with respiratory, gastrointestinal, and/or neurological diseases. There is some evidence that suggest that human respiratory CoVs may show neuroinvasive properties. Indeed, the severe acute respiratory syndrome coronavirus (SARS-CoV), causing severe acute respiratory syndrome, and the CoVs OC43 and 229E were found in the brains of SARS patients and multiple sclerosis patients, respectively. These findings suggest that hematogenously spread CoVs may gain access to the CNS at the BBB level. Herein we report for the first time that CoVs exhibit the ability to cross the BBB according to strain virulence. BBB invasion by CoVs correlates with virus-induced disruption of tight junctions on BMECs, leading to BBB dysfunction and enhanced permeability. We provide evidence that production of IFN-β by BMECs during CoV infection may prevent BBB breakdown and brain viral invasion.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

