Association of HIV-1 Envelope-Specific Breast Milk IgA Responses with Reduced Risk of Postnatal Mother-to-Child Transmission of HIV-1
- PMID: 26202232
- PMCID: PMC4577885
- DOI: 10.1128/JVI.01560-15
Association of HIV-1 Envelope-Specific Breast Milk IgA Responses with Reduced Risk of Postnatal Mother-to-Child Transmission of HIV-1
Abstract
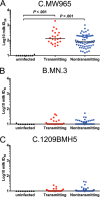
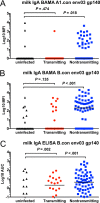
Infants born to HIV-1-infected mothers in resource-limited areas where replacement feeding is unsafe and impractical are repeatedly exposed to HIV-1 throughout breastfeeding. Despite this, the majority of infants do not contract HIV-1 postnatally, even in the absence of maternal antiretroviral therapy. This suggests that immune factors in breast milk of HIV-1-infected mothers help to limit vertical transmission. We compared the HIV-1 envelope-specific breast milk and plasma antibody responses of clade C HIV-1-infected postnatally transmitting and nontransmitting mothers in the control arm of the Malawi-based Breastfeeding Antiretrovirals and Nutrition Study using multivariable logistic regression modeling. We found no association between milk or plasma neutralization activity, antibody-dependent cell-mediated cytotoxicity, or HIV-1 envelope-specific IgG responses and postnatal transmission risk. While the envelope-specific breast milk and plasma IgA responses also did not reach significance in predicting postnatal transmission risk in the primary model after correction for multiple comparisons, subsequent exploratory analysis using two distinct assay methodologies demonstrated that the magnitudes of breast milk total and secretory IgA responses against a consensus HIV-1 envelope gp140 (B.con env03) were associated with reduced postnatal transmission risk. These results suggest a protective role for mucosal HIV-1 envelope-specific IgA responses in the context of postnatal virus transmission. This finding supports further investigations into the mechanisms by which mucosal IgA reduces risk of HIV-1 transmission via breast milk and into immune interventions aimed at enhancing this response.
Importance: Infants born to HIV-1-infected mothers are repeatedly exposed to the virus in breast milk. Remarkably, the transmission rate is low, suggesting that immune factors in the breast milk of HIV-1-infected mothers help to limit transmission. We compared the antibody responses in plasma and breast milk of HIV-1-transmitting and -nontransmitting mothers to identify responses that correlated with reduced risk of postnatal HIV-1 transmission. We found that neither plasma nor breast milk IgG antibody responses were associated with risk of HIV-1 transmission. In contrast, the magnitudes of the breast milk IgA and secretory IgA responses against HIV-1 envelope proteins were associated with reduced risk of postnatal HIV-1 transmission. The results of this study support further investigations of the mechanisms by which mucosal IgA may reduce the risk of HIV-1 transmission via breastfeeding and the development of strategies to enhance milk envelope-specific IgA responses to reduce mother-to-child HIV transmission and promote an HIV-free generation.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Maternal Humoral Immune Responses Do Not Predict Postnatal HIV-1 Transmission Risk in Antiretroviral-Treated Mothers from the IMPAACT PROMISE Study.mSphere. 2019 Oct 23;4(5):e00716-19. doi: 10.1128/mSphere.00716-19. mSphere. 2019. PMID: 31645430 Free PMC article. Clinical Trial.
-
Combined HIV-1 Envelope Systemic and Mucosal Immunization of Lactating Rhesus Monkeys Induces a Robust Immunoglobulin A Isotype B Cell Response in Breast Milk.J Virol. 2016 Apr 29;90(10):4951-4965. doi: 10.1128/JVI.00335-16. Print 2016 May 15. J Virol. 2016. PMID: 26937027 Free PMC article.
-
Maternal but Not Infant Anti-HIV-1 Neutralizing Antibody Response Associates with Enhanced Transmission and Infant Morbidity.mBio. 2017 Oct 24;8(5):e01373-17. doi: 10.1128/mBio.01373-17. mBio. 2017. PMID: 29066544 Free PMC article.
-
Antiretroviral drugs to prevent mother-to-child transmission of HIV during breastfeeding.Curr HIV Res. 2013 Mar;11(2):102-25. doi: 10.2174/1570162x11311020004. Curr HIV Res. 2013. PMID: 23432487 Review.
-
Transfer of antibody via mother's milk.Vaccine. 2003 Jul 28;21(24):3374-6. doi: 10.1016/s0264-410x(03)00336-0. Vaccine. 2003. PMID: 12850343 Review.
Cited by
-
Humoral Immune Correlates for Prevention of Postnatal Cytomegalovirus Acquisition.J Infect Dis. 2019 Jul 31;220(5):772-780. doi: 10.1093/infdis/jiz192. J Infect Dis. 2019. PMID: 31107951 Free PMC article.
-
Pentavalent HIV-1 vaccine protects against simian-human immunodeficiency virus challenge.Nat Commun. 2017 Jun 8;8:15711. doi: 10.1038/ncomms15711. Nat Commun. 2017. PMID: 28593989 Free PMC article.
-
Research on Maternal Vaccination for HIV Prevention.Clin Perinatol. 2024 Dec;51(4):769-782. doi: 10.1016/j.clp.2024.08.007. Epub 2024 Sep 11. Clin Perinatol. 2024. PMID: 39487019 Review.
-
A heterologous prime-boosting strategy with replicating Vaccinia virus vectors and plant-produced HIV-1 Gag/dgp41 virus-like particles.Virology. 2017 Jul;507:242-256. doi: 10.1016/j.virol.2017.04.008. Epub 2017 Apr 28. Virology. 2017. PMID: 28458036 Free PMC article.
-
Polyclonal HIV envelope-specific breast milk antibodies limit founder SHIV acquisition and cell-associated virus loads in infant rhesus monkeys.Mucosal Immunol. 2018 Nov;11(6):1716-1726. doi: 10.1038/s41385-018-0067-7. Epub 2018 Aug 16. Mucosal Immunol. 2018. PMID: 30115994 Free PMC article.
References
-
- UNAIDS. 2013. Global report: UNAIDS report on the global AIDS epidemic 2013. Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Global_Repo....
-
- WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. 2000. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet 355:451–455. doi:10.1016/S0140-6736(00)82011-5. - DOI - PubMed
-
- Breastfeeding and HIV International Transmission Study Group, Coutsoudis A, Dabis F, Fawzi W, Gaillard P, Haverkamp G, Harris DR, Jackson JB, Leroy V, Meda N, Msellati P, Newell ML, Nsuati R, Read JS, Wiktor S. 2004. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis 189:2154–2166. doi:10.1086/420834. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI100645/AI/NIAID NIH HHS/United States
- R24 TW00798/TW/FIC NIH HHS/United States
- SIP 26-04 U48-DP000059-01/DP/NCCDPHP CDC HHS/United States
- AI100645/AI/NIAID NIH HHS/United States
- U48DP001944/ACL/ACL HHS/United States
- U19 AI067854/AI/NIAID NIH HHS/United States
- 5P30AI064518/AI/NIAID NIH HHS/United States
- AI06380/AI/NIAID NIH HHS/United States
- U48 DP000059/DP/NCCDPHP CDC HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- SIP 22-09 U48-DP001944-01/DP/NCCDPHP CDC HHS/United States
- U48 DP001944/DP/NCCDPHP CDC HHS/United States
- P30 AI027767/AI/NIAID NIH HHS/United States
- P30-AI50410/AI/NIAID NIH HHS/United States
- DHHS/NIH/FIC 2-D43 TW01039-06/TW/FIC NIH HHS/United States
- 5P30 AI050410/AI/NIAID NIH HHS/United States
- R01 AI106380/AI/NIAID NIH HHS/United States
- P30 AI064518/AI/NIAID NIH HHS/United States
- SIP 13-01U48-CCU409660-09/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

