The Epstein-Barr Virus BDLF4 Gene Is Required for Efficient Expression of Viral Late Lytic Genes
- PMID: 26202235
- PMCID: PMC4577904
- DOI: 10.1128/JVI.01604-15
The Epstein-Barr Virus BDLF4 Gene Is Required for Efficient Expression of Viral Late Lytic Genes
Abstract
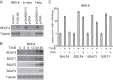
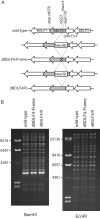
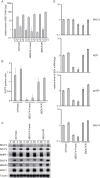
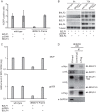
Epstein-Barr virus (EBV) is a gammaherpesvirus, associated with infectious mononucleosis and various types of malignancy. We focused here on the BDLF4 gene of EBV and identified it as a lytic gene, expressed with early kinetics. Viral late gene expression of the BDLF4 knockout strain was severely restricted; this could be restored by an exogenous supply of BDLF4. These results indicate that BDLF4 is important for the EBV lytic replication cycle, especially in late gene expression.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Epstein-Barr Virus BKRF4 Gene Product Is Required for Efficient Progeny Production.J Virol. 2017 Nov 14;91(23):e00975-17. doi: 10.1128/JVI.00975-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28904200 Free PMC article.
-
S-Like-Phase Cyclin-Dependent Kinases Stabilize the Epstein-Barr Virus BDLF4 Protein To Temporally Control Late Gene Transcription.J Virol. 2019 Apr 3;93(8):e01707-18. doi: 10.1128/JVI.01707-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30700607 Free PMC article.
-
Roles of Epstein-Barr virus BGLF3.5 gene and two upstream open reading frames in lytic viral replication in HEK293 cells.Virology. 2015 Sep;483:44-53. doi: 10.1016/j.virol.2015.04.007. Epub 2015 May 15. Virology. 2015. PMID: 25965794
-
Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases.Autoimmunity. 2008 May;41(4):298-328. doi: 10.1080/08916930802024772. Autoimmunity. 2008. PMID: 18432410 Review.
-
Lytic cycle switches of oncogenic human gammaherpesviruses.Adv Cancer Res. 2007;97:81-109. doi: 10.1016/S0065-230X(06)97004-3. Adv Cancer Res. 2007. PMID: 17419942 Review.
Cited by
-
Herpesvirus Late Gene Expression: A Viral-Specific Pre-initiation Complex Is Key.Front Microbiol. 2016 Jun 6;7:869. doi: 10.3389/fmicb.2016.00869. eCollection 2016. Front Microbiol. 2016. PMID: 27375590 Free PMC article. Review.
-
Lytic Replication and Reactivation from B Cells Is Not Required for Establishing or Maintaining Gammaherpesvirus Latency In Vivo.J Virol. 2022 Jun 22;96(12):e0069022. doi: 10.1128/jvi.00690-22. Epub 2022 Jun 1. J Virol. 2022. PMID: 35647668 Free PMC article.
-
Comparative Analysis of the Humoral Immune Response to the EBV Proteome across EBV-Related Malignancies.Cancer Epidemiol Biomarkers Prev. 2023 May 1;32(5):687-696. doi: 10.1158/1055-9965.EPI-22-0452. Cancer Epidemiol Biomarkers Prev. 2023. PMID: 36788424 Free PMC article.
-
Antitumor activity of cyclin-dependent kinase inhibitor alsterpaullone in Epstein-Barr virus-associated lymphoproliferative disorders.Cancer Sci. 2020 Jan;111(1):279-287. doi: 10.1111/cas.14241. Epub 2019 Dec 11. Cancer Sci. 2020. PMID: 31743514 Free PMC article.
-
The C-Terminus of Epstein-Barr Virus BRRF2 Is Required for its Proper Localization and Efficient Virus Production.Front Microbiol. 2017 Jan 31;8:125. doi: 10.3389/fmicb.2017.00125. eCollection 2017. Front Microbiol. 2017. PMID: 28197146 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

