Protection from Severe Influenza Virus Infections in Mice Carrying the Mx1 Influenza Virus Resistance Gene Strongly Depends on Genetic Background
- PMID: 26202236
- PMCID: PMC4577889
- DOI: 10.1128/JVI.01305-15
Protection from Severe Influenza Virus Infections in Mice Carrying the Mx1 Influenza Virus Resistance Gene Strongly Depends on Genetic Background
Abstract
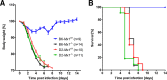
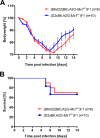
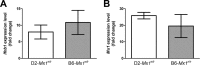
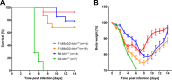
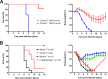
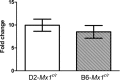
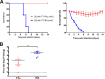
Influenza virus infections represent a serious threat to human health. Both extrinsic and intrinsic factors determine the severity of influenza. The MX dynamin-like GTPase 1 (Mx1) gene has been shown to confer strong resistance to influenza A virus infections in mice. Most laboratory mouse strains, including C57BL/6J, carry nonsense or deletion mutations in Mx1 and thus a nonfunctional allele, whereas wild-derived mouse strains carry a wild-type Mx1 allele. Congenic C57BL/6J (B6-Mx1(r/r)) mice expressing a wild-type allele from the A2G mouse strain are highly resistant to influenza A virus infections, to both mono- and polybasic subtypes. Furthermore, in genetic mapping studies, Mx1 was identified as the major locus of resistance to influenza virus infections. Here, we investigated whether the Mx1 protective function is influenced by the genetic background. For this, we generated a congenic mouse strain carrying the A2G wild-type Mx1 resistance allele on a DBA/2J background (D2-Mx1(r/r)). Most remarkably, congenic D2-Mx1(r/r) mice expressing a functional Mx1 wild-type allele are still highly susceptible to H1N1 virus. However, pretreatment of D2-Mx1(r/r) mice with alpha interferon protected them from lethal infections. Our results showed, for the first time, that the presence of an Mx1 wild-type allele from A2G as such does not fully protect mice from lethal influenza A virus infections. These observations are also highly relevant for susceptibility to influenza virus infections in humans.
Importance: Influenza A virus represents a major health threat to humans. Seasonal influenza epidemics cause high economic loss, morbidity, and deaths each year. Genetic factors of the host strongly influence susceptibility and resistance to virus infections. The Mx1 (MX dynamin-like GTPase 1) gene has been described as a major resistance gene in mice and humans. Most inbred laboratory mouse strains are deficient in Mx1, but congenic B6-Mx1(r/r) mice that carry the wild-type Mx1 gene from the A2G mouse strain are highly resistant. Here, we show that, very unexpectedly, congenic D2-Mx1(r/r) mice carrying the wild-type Mx1 gene from the A2G strain are not fully protected against lethal influenza virus infections. These observations demonstrate that the genetic background is very important for the protective function of the Mx1 resistance gene. Our results are also highly relevant for understanding genetic susceptibility to influenza virus infections in humans.
Copyright © 2015, Shin et al.
Figures


Similar articles
-
Differential lung gene expression changes in C57BL/6 and DBA/2 mice carrying an identical functional Mx1 gene reveals crucial differences in the host response.BMC Genom Data. 2024 Feb 15;25(1):19. doi: 10.1186/s12863-024-01203-3. BMC Genom Data. 2024. PMID: 38360537 Free PMC article.
-
Influenza Virus Susceptibility of Wild-Derived CAST/EiJ Mice Results from Two Amino Acid Changes in the MX1 Restriction Factor.J Virol. 2016 Nov 14;90(23):10682-10692. doi: 10.1128/JVI.01213-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27654285 Free PMC article.
-
Generation of congenic mouse strains by introducing the virus-resistant genes, Mx1 and Oas1b, of feral mouse-derived inbred strain MSM/Ms into the common strain C57BL/6J.Jpn J Vet Res. 2009 Aug;57(2):89-99. Jpn J Vet Res. 2009. PMID: 19827744
-
Mx genes: host determinants controlling influenza virus infection and trans-species transmission.Hum Genet. 2020 Jun;139(6-7):695-705. doi: 10.1007/s00439-019-02092-8. Epub 2019 Nov 26. Hum Genet. 2020. PMID: 31773252 Free PMC article. Review.
-
Host genetics of severe influenza: from mouse Mx1 to human IRF7.Curr Opin Immunol. 2016 Feb;38:109-20. doi: 10.1016/j.coi.2015.12.002. Epub 2016 Jan 4. Curr Opin Immunol. 2016. PMID: 26761402 Free PMC article. Review.
Cited by
-
Exercise, immune function and respiratory infection: An update on the influence of training and environmental stress.Immunol Cell Biol. 2016 Feb;94(2):132-9. doi: 10.1038/icb.2015.99. Epub 2015 Nov 13. Immunol Cell Biol. 2016. PMID: 26563736 Review.
-
Time-dependent viral interference between influenza virus and coronavirus in the infection of differentiated porcine airway epithelial cells.Virulence. 2021 Dec;12(1):1111-1121. doi: 10.1080/21505594.2021.1911148. Virulence. 2021. PMID: 34034617 Free PMC article.
-
New polymorphism of the influenza virus resistance Mx1 gene in Iberian domestic pigs.Postdoc J. 2016 Sep;4(9):15-19. doi: 10.14304/surya.jpr.v4n9.3. Postdoc J. 2016. PMID: 27774491 Free PMC article.
-
The differentiated airway epithelium infected by influenza viruses maintains the barrier function despite a dramatic loss of ciliated cells.Sci Rep. 2016 Dec 22;6:39668. doi: 10.1038/srep39668. Sci Rep. 2016. PMID: 28004801 Free PMC article.
-
Peptidylarginine Deiminase 2 in Murine Antiviral and Autoimmune Antibody Responses.J Immunol Res. 2022 Jan 17;2022:5258221. doi: 10.1155/2022/5258221. eCollection 2022. J Immunol Res. 2022. PMID: 35083342 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

