Reduced Simian Immunodeficiency Virus Replication in Macrophages of Sooty Mangabeys Is Associated with Increased Expression of Host Restriction Factors
- PMID: 26202248
- PMCID: PMC4580171
- DOI: 10.1128/JVI.00710-15
Reduced Simian Immunodeficiency Virus Replication in Macrophages of Sooty Mangabeys Is Associated with Increased Expression of Host Restriction Factors
Abstract
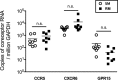
Macrophages are target cells of HIV/SIV infection that may play a role in AIDS pathogenesis and contribute to the long-lived reservoir of latently infected cells during antiretroviral therapy (ART). In previous work, we and others have shown that during pathogenic SIV infection of rhesus macaques (RMs), rapid disease progression is associated with high levels of in vivo macrophage infection. In contrast, during nonpathogenic SIV infection of sooty mangabeys (SMs), neither spontaneous nor experimental CD4(+) T cell depletion results in substantial levels of in vivo macrophage infection. To test the hypothesis that SM macrophages are intrinsically more resistant to SIV infection than RM macrophages, we undertook an in vitro comparative assessment of monocyte-derived macrophages (MDMs) from both nonhuman primate species. Using the primary isolate SIVM949, which replicates well in lymphocytes from both RMs and SMs, we found that infection of RM macrophages resulted in persistent SIV-RNA production while SIV-RNA levels in SM macrophage cultures decreased 10- to 100-fold over a similar temporal course of in vitro infection. To explore potential mechanisms responsible for the lower levels of SIV replication and/or production in macrophages from SMs we comparatively assessed, in the two studied species, the expression of the SIV coreceptor as well as the expression of a number of host restriction factors. While previous studies showed that SM monocytes express lower levels of CCR5 (but not CD4) than RM monocytes, the level of CCR5 expression in MDMs was similar in the two species. Interestingly, we found that SM macrophages exhibited a significantly greater increase in the expression of tetherin (P = 0.003) and TRIM22 (P = 0.0006) in response to alpha interferon stimulation and increased expression of multiple host restriction factors in response to lipopolysaccharide stimulation and exposure to SIV. Overall, these findings confirm, in an in vitro infection system, that SM macrophages are relatively more resistant to SIV infection compared to RM macrophages, and suggest that a combination of entry and postentry restriction mechanisms may protect these cells from productive SIV infection.
Importance: This manuscript represents the first in vivo comparative analysis of monocyte-derived macrophages (MDMs) between rhesus macaques, i.e., experimental SIV hosts in which the infection is pathogenic and macrophages can be infected, and sooty mangabeys, i.e., natural SIV hosts in which the infection is nonpathogenic and macrophages are virtually never infected in vivo. This study demonstrates that mangabey-derived MDMs are more resistant to SIV infection in vitro compared to macaque-derived MDMs, and provides a potential explanation for this observation by showing increased expression of specific retrovirus restriction factors in mangabey-derived macrophages. Overall, this study is important as it contributes to our understanding of why SIV infection is nonpathogenic in sooty mangabeys while it is pathogenic in macaques, and is consistent with a pathogenic role for in vivo macrophage infection during pathogenic lentiviral infection.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





Similar articles
-
Dualtropic CXCR6/CCR5 Simian Immunodeficiency Virus (SIV) Infection of Sooty Mangabey Primary Lymphocytes: Distinct Coreceptor Use in Natural versus Pathogenic Hosts of SIV.J Virol. 2015 Sep;89(18):9252-61. doi: 10.1128/JVI.01236-15. Epub 2015 Jun 24. J Virol. 2015. PMID: 26109719 Free PMC article.
-
Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts.J Virol. 2005 Apr;79(7):4043-54. doi: 10.1128/JVI.79.7.4043-4054.2005. J Virol. 2005. PMID: 15767406 Free PMC article.
-
Depletion of CD8+ cells in sooty mangabey monkeys naturally infected with simian immunodeficiency virus reveals limited role for immune control of virus replication in a natural host species.J Immunol. 2007 Jun 15;178(12):8002-12. doi: 10.4049/jimmunol.178.12.8002. J Immunol. 2007. PMID: 17548637
-
Naturally SIV-infected sooty mangabeys: are we closer to understanding why they do not develop AIDS?J Med Primatol. 2005 Oct;34(5-6):243-52. doi: 10.1111/j.1600-0684.2005.00122.x. J Med Primatol. 2005. PMID: 16128919 Review.
-
SIV Coreceptor Specificity in Natural and Non-Natural Host Infection: Implications for Cell Targeting and Differential Outcomes from Infection.Curr HIV Res. 2018;16(1):41-51. doi: 10.2174/1570162X15666171124121805. Curr HIV Res. 2018. PMID: 29173179 Review.
Cited by
-
Nonhuman Primate Models and Understanding the Pathogenesis of HIV Infection and AIDS.ILAR J. 2017 Dec 1;58(2):160-171. doi: 10.1093/ilar/ilx032. ILAR J. 2017. PMID: 29228218 Free PMC article.
-
HIV-2/SIV Vpx antagonises NF-κB activation by targeting p65.Retrovirology. 2022 Jan 24;19(1):2. doi: 10.1186/s12977-021-00586-w. Retrovirology. 2022. PMID: 35073912 Free PMC article.
-
Sooty mangabey genome sequence provides insight into AIDS resistance in a natural SIV host.Nature. 2018 Jan 3;553(7686):77-81. doi: 10.1038/nature25140. Nature. 2018. PMID: 29300007 Free PMC article.
-
Treating the host response to emerging virus diseases: lessons learned from sepsis, pneumonia, influenza and Ebola.Ann Transl Med. 2016 Nov;4(21):421. doi: 10.21037/atm.2016.11.03. Ann Transl Med. 2016. PMID: 27942512 Free PMC article. Review.
-
HIV Latency in Myeloid Cells: Challenges for a Cure.Pathogens. 2022 May 24;11(6):611. doi: 10.3390/pathogens11060611. Pathogens. 2022. PMID: 35745465 Free PMC article. Review.
References
-
- Nicholson JK, Cross GD, Callaway CS, McDougal JS. 1986. In vitro infection of human monocytes with human T lymphotropic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV). J Immunol 137:323–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

