Blockade of Wnt/β-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype
- PMID: 26202299
- PMCID: PMC5378883
- DOI: 10.1038/srep12465
Blockade of Wnt/β-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype
Abstract
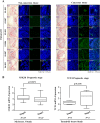
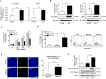
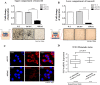
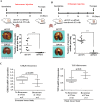
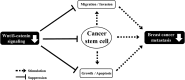
The identification of cancer stem cells (CSCs) represents an important milestone in the understanding of chemodrug resistance and cancer recurrence. More specifically, some studies have suggested that potential metastasis-initiating cells (MICs) might be present within small CSC populations. The targeting and eradication of these cells represents a potential strategy for significantly improving clinical outcomes. A number of studies have suggested that dysregulation of Wnt/β-catenin signaling occurs in human breast cancer. Consistent with these findings, our previous data have shown that the relative level of Wnt/β-catenin signaling activity in breast cancer stem cells (BCSCs) is significantly higher than that in bulk cancer cells. These results suggest that BCSCs could be sensitive to therapeutic approaches targeting Wnt/β-catenin signaling pathway. In this context, abnormal Wnt/β-catenin signaling activity may be an important clinical feature of breast cancer and a predictor of poor survival. We therefore hypothesized that Wnt/β-catenin signaling might regulate self-renewal and CSC migration, thereby enabling metastasis and systemic tumor dissemination in breast cancer. Here, we investigated the effects of inhibiting Wnt/β-catenin signaling on cancer cell migratory potential by examining the expression of CSC-related genes, and we examined how this pathway links metastatic potential with tumor formation in vitro and in vivo.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
IWR-1, a tankyrase inhibitor, attenuates Wnt/β-catenin signaling in cancer stem-like cells and inhibits in vivo the growth of a subcutaneous human osteosarcoma xenograft.Cancer Lett. 2018 Feb 1;414:1-15. doi: 10.1016/j.canlet.2017.11.004. Epub 2017 Nov 8. Cancer Lett. 2018. PMID: 29126913
-
In vivo β-catenin attenuation by the integrin α5-targeting nano-delivery strategy suppresses triple negative breast cancer stemness and metastasis.Biomaterials. 2019 Jan;188:160-172. doi: 10.1016/j.biomaterials.2018.10.019. Epub 2018 Oct 18. Biomaterials. 2019. PMID: 30352320
-
Aryl hydrocarbon receptor/cytochrome P450 1A1 pathway mediates breast cancer stem cells expansion through PTEN inhibition and β-Catenin and Akt activation.Mol Cancer. 2017 Jan 19;16(1):14. doi: 10.1186/s12943-016-0570-y. Mol Cancer. 2017. PMID: 28103884 Free PMC article.
-
Development of Small Molecules Targeting the Wnt Signaling Pathway in Cancer Stem Cells for the Treatment of Colorectal Cancer.Clin Colorectal Cancer. 2015 Sep;14(3):133-45. doi: 10.1016/j.clcc.2015.02.001. Epub 2015 Feb 17. Clin Colorectal Cancer. 2015. PMID: 25799881 Review.
-
Targeting the Wnt/β-catenin signaling pathway in cancer.J Hematol Oncol. 2020 Dec 4;13(1):165. doi: 10.1186/s13045-020-00990-3. J Hematol Oncol. 2020. PMID: 33276800 Free PMC article. Review.
Cited by
-
The Effect of Adipocyte-Secreted Factors in Activating Focal Adhesion Kinase-Mediated Cell Signaling Pathway towards Metastasis in Breast Cancer Cells.Int J Mol Sci. 2023 Nov 22;24(23):16605. doi: 10.3390/ijms242316605. Int J Mol Sci. 2023. PMID: 38068928 Free PMC article.
-
Cancer stem cells and strategies for targeted drug delivery.Drug Deliv Transl Res. 2021 Oct;11(5):1779-1805. doi: 10.1007/s13346-020-00863-9. Epub 2020 Oct 23. Drug Deliv Transl Res. 2021. PMID: 33095384 Free PMC article. Review.
-
Cancer Stem Cells: An Ever-Hiding Foe.Exp Suppl. 2022;113:219-251. doi: 10.1007/978-3-030-91311-3_8. Exp Suppl. 2022. PMID: 35165866
-
The Significance of Cancer Stem Cells and Epithelial-Mesenchymal Transition in Metastasis and Anti-Cancer Therapy.Int J Mol Sci. 2023 Jan 29;24(3):2555. doi: 10.3390/ijms24032555. Int J Mol Sci. 2023. PMID: 36768876 Free PMC article. Review.
-
MicroRNAs Involved in Carcinogenesis, Prognosis, Therapeutic Resistance and Applications in Human Triple-Negative Breast Cancer.Cells. 2019 Nov 22;8(12):1492. doi: 10.3390/cells8121492. Cells. 2019. PMID: 31766744 Free PMC article. Review.
References
-
- Pang R. et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell 6, 603–15 (2010). - PubMed
-
- Hermann P.C. et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1, 313–23 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

