Arnebin-1 promotes angiogenesis by inducing eNOS, VEGF and HIF-1α expression through the PI3K-dependent pathway
- PMID: 26202335
- PMCID: PMC4533782
- DOI: 10.3892/ijmm.2015.2292
Arnebin-1 promotes angiogenesis by inducing eNOS, VEGF and HIF-1α expression through the PI3K-dependent pathway
Retraction in
-
[Retracted] Arnebin‑1 promotes angiogenesis by inducing eNOS, VEGF and HIF‑1α expression through the PI3K‑dependent pathway.Int J Mol Med. 2023 May;51(5):38. doi: 10.3892/ijmm.2023.5241. Epub 2023 Apr 7. Int J Mol Med. 2023. PMID: 37026507 Free PMC article.
Abstract
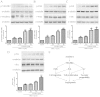
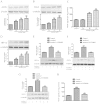
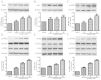
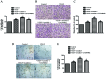
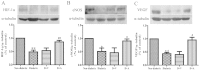
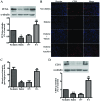
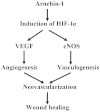
Arnebin-1, a naphthoquinone derivative, plays a crucial role in the wound healing properties of Zicao (a traditional wound healing herbal medicine). It has been noted that Arnebin-1, in conjunction with vascular endothelial growth factor (VEGF), exerts a synergistic pro-angiogenic effect on human umbilical vein endothelial cells (HUVECs) and accelerates the healing process of diabetic wounds. However, the mechanisms responsible for the pro-angiogenic effect of arnebin‑1 on HUVECs and its healing effect on diabetic wounds have not yet been fully elucidated. In this study, in an aim to elucidate these mechanisms of action of arnebin‑1, we investigated the effects of arnebin‑1 on the VEGF receptor 2 (VEGFR2) and the phosphoinositide 3-kinase (PI3K)‑dependent signaling pathways in HUVECs treated with VEGF by western blot analysis. The pro‑angiogenic effects of arnebin‑1 on HUVECs, including its effects on proliferation and migration, were evaluated by MTT assay, Transwell assay and tube formation assay in vitro. The expression levels of hypoxia-inducible factor (HIF)‑1α, endothelial nitric oxide synthase (eNOS) and VEGF were determined by western blot analysis in the HUVECs and wound tissues obtained from non‑diabetic and diabetic rats. CD31 expression in the rat wounds was evaluated by immunofluorescence staining. We found that the activation of the VEGFR2 signaling pathway induced by VEGF was enhanced by arnebin‑1. Arnebin‑1 promoted endothelial cell proliferation, migration and tube formation through the PI3K‑dependent pathway. Moreover, Arnebin‑1 significantly increased the eNOS, VEGF and HIF‑1α expression levels in the HUVECs and accelerated the healing of diabetic wounds through the PI3K‑dependent signaling pathway. CD31 expression was markedly enhanced in the wounds of diabetic rats treated with arnebin‑1 compared to the wounds of untreated diabetic rats. Therefore, the findings of the present study indicate that arnebin-1 promotes the wound healing process in diabetic rats by eliciting a pro-angiogenic response.
Figures

Similar articles
-
Arnebin-1 promotes the angiogenesis of human umbilical vein endothelial cells and accelerates the wound healing process in diabetic rats.J Ethnopharmacol. 2014 Jul 3;154(3):653-62. doi: 10.1016/j.jep.2014.04.038. Epub 2014 May 1. J Ethnopharmacol. 2014. PMID: 24794013
-
Total Flavones of Abelmoschus manihot Exhibits Pro-Angiogenic Activity by Activating the VEGF-A/VEGFR2-PI3K/Akt Signaling Axis.Am J Chin Med. 2018;46(3):567-583. doi: 10.1142/S0192415X18500295. Epub 2018 Mar 29. Am J Chin Med. 2018. PMID: 29595071
-
Notoginsenoside Ft1 promotes angiogenesis via HIF-1α mediated VEGF secretion and the regulation of PI3K/AKT and Raf/MEK/ERK signaling pathways.Biochem Pharmacol. 2012 Sep 15;84(6):784-92. doi: 10.1016/j.bcp.2012.05.024. Epub 2012 Jul 4. Biochem Pharmacol. 2012. PMID: 22771629
-
Lipid nanoparticle: advanced drug delivery systems for promotion of angiogenesis in diabetic wounds.J Liposome Res. 2025 Mar;35(1):76-85. doi: 10.1080/08982104.2024.2378962. Epub 2024 Jul 15. J Liposome Res. 2025. PMID: 39007863 Review.
-
Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective.Int J Mol Sci. 2023 Feb 27;24(5):4607. doi: 10.3390/ijms24054607. Int J Mol Sci. 2023. PMID: 36902038 Free PMC article. Review.
Cited by
-
A novel family of small molecule HIF-1 alpha stabilizers for the treatment of diabetic wounds; an integrated in silico, in vitro, and in vivo strategy.RSC Adv. 2022 Nov 1;12(48):31293-31302. doi: 10.1039/d2ra05364k. eCollection 2022 Oct 27. RSC Adv. 2022. PMID: 36349012 Free PMC article.
-
A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective.Int J Mol Sci. 2022 Aug 24;23(17):9573. doi: 10.3390/ijms23179573. Int J Mol Sci. 2022. PMID: 36076971 Free PMC article. Review.
-
Expressions of heat shock protein 90, inducible nitric oxide synthase, and vascular endothelial growth factor in the skin of diabetic rats.Vet World. 2021 Jul;14(7):1804-1807. doi: 10.14202/vetworld.2021.1804-1807. Epub 2021 Jul 13. Vet World. 2021. PMID: 34475701 Free PMC article.
-
Targeting Signalling Pathways in Chronic Wound Healing.Int J Mol Sci. 2023 Dec 19;25(1):50. doi: 10.3390/ijms25010050. Int J Mol Sci. 2023. PMID: 38203220 Free PMC article. Review.
-
Glucobrassicin Metabolites Ameliorate the Development of Portal Hypertension and Cirrhosis in Bile Duct-Ligated Rats.Int J Mol Sci. 2019 Aug 26;20(17):4161. doi: 10.3390/ijms20174161. Int J Mol Sci. 2019. PMID: 31454890 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

