PKR inhibits the DNA damage response, and is associated with poor survival in AML and accelerated leukemia in NHD13 mice
- PMID: 26202421
- PMCID: PMC4582335
- DOI: 10.1182/blood-2015-03-635227
PKR inhibits the DNA damage response, and is associated with poor survival in AML and accelerated leukemia in NHD13 mice
Abstract
Increased expression of the interferon-inducible double-stranded RNA-activated protein kinase (PKR) has been reported in acute leukemia and solid tumors, but the role of PKR has been unclear. Now, our results indicate that high PKR expression in CD34(+) cells of acute myeloid leukemia (AML) patients correlates with worse survival and shortened remission duration. Significantly, we find that PKR has a novel and previously unrecognized nuclear function to inhibit DNA damage response signaling and double-strand break repair. Nuclear PKR antagonizes ataxia-telangiectasia mutated (ATM) activation by a mechanism dependent on protein phosphatase 2A activity. Thus, inhibition of PKR expression or activity promotes ATM activation, γ-H2AX formation, and phosphorylation of NBS1 following ionizing irradiation. PKR transgenic but not PKR null mice demonstrate a mutator phenotype characterized by radiation-induced and age-associated genomic instability that was partially reversed by short-term pharmacologic PKR inhibition. Furthermore, the age-associated accumulation of somatic mutations that occurs in the Nup98-HOXD13 (NHD13) mouse model of leukemia progression was significantly elevated by co-expression of a PKR transgene, whereas knockout of PKR expression or pharmacologic inhibition of PKR activity reduced the frequency of spontaneous mutations in vivo. Thus, PKR cooperated with the NHD13 transgene to accelerate leukemia progression and shorten survival. Taken together, these results indicate that increased nuclear PKR has an oncogenic function that promotes the accumulation of potentially deleterious mutations. Thus, PKR inhibition may be a therapeutically useful strategy to prevent leukemia progression or relapse, and improve clinical outcomes.
© 2015 by The American Society of Hematology.
Figures


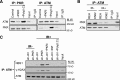
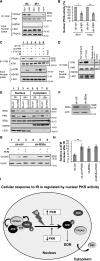
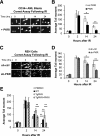
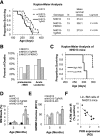
Comment in
-
Nuclear, not cytoplasmic, PKR maneuvers in AML.Blood. 2015 Sep 24;126(13):1523-4. doi: 10.1182/blood-2015-08-661421. Blood. 2015. PMID: 26405214
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

