Positive and negative regulation by SLP-76/ADAP and Pyk2 of chemokine-stimulated T-lymphocyte adhesion mediated by integrin α4β1
- PMID: 26202465
- PMCID: PMC4569313
- DOI: 10.1091/mbc.E14-07-1246
Positive and negative regulation by SLP-76/ADAP and Pyk2 of chemokine-stimulated T-lymphocyte adhesion mediated by integrin α4β1
Abstract
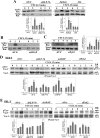
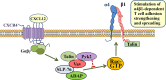
Stimulation by chemokines of integrin α4β1-dependent T-lymphocyte adhesion is a crucial step for lymphocyte trafficking. The adaptor Vav1 is required for chemokine-activated T-cell adhesion mediated by α4β1. Conceivably, proteins associating with Vav1 could potentially modulate this adhesion. Correlating with activation by the chemokine CXCL12 of T-lymphocyte attachment to α4β1 ligands, a transient stimulation in the association of Vav1 with SLP-76, Pyk2, and ADAP was observed. Using T-cells depleted for SLP-76, ADAP, or Pyk2, or expressing Pyk2 kinase-inactive forms, we show that SLP-76 and ADAP stimulate chemokine-activated, α4β1-mediated adhesion, whereas Pyk2 opposes T-cell attachment. While CXCL12-promoted generation of high-affinity α4β1 is independent of SLP-76, ADAP, and Pyk2, the strength of α4β1-VCAM-1 interaction and cell spreading on VCAM-1 are targets of regulation by these three proteins. GTPase assays, expression of activated or dominant-negative Rac1, or combined ADAP and Pyk2 silencing indicated that Rac1 activation by CXCL12 is a common mediator response in SLP-76-, ADAP-, and Pyk2-regulated cell adhesion involving α4β1. Our data strongly suggest that chemokine-stimulated associations between Vav1, SLP-76, and ADAP facilitate Rac1 activation and α4β1-mediated adhesion, whereas Pyk2 opposes this adhesion by limiting Rac1 activation.
© 2015 Dios-Esponera, Isern de Val, et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures


Similar articles
-
Vav1 and Rac control chemokine-promoted T lymphocyte adhesion mediated by the integrin alpha4beta1.Mol Biol Cell. 2005 Jul;16(7):3223-35. doi: 10.1091/mbc.e04-12-1049. Epub 2005 May 4. Mol Biol Cell. 2005. PMID: 15872091 Free PMC article.
-
DOCK2 is required for chemokine-promoted human T lymphocyte adhesion under shear stress mediated by the integrin alpha4beta1.J Immunol. 2006 Oct 15;177(8):5215-25. doi: 10.4049/jimmunol.177.8.5215. J Immunol. 2006. PMID: 17015707
-
Chemokine-induced Zap70 kinase-mediated dissociation of the Vav1-talin complex activates alpha4beta1 integrin for T cell adhesion.Immunity. 2009 Dec 18;31(6):953-64. doi: 10.1016/j.immuni.2009.09.021. Epub 2009 Dec 10. Immunity. 2009. PMID: 20005136
-
ADAP-ting TCR signaling to integrins.Sci STKE. 2002 Apr 9;2002(127):re3. doi: 10.1126/stke.2002.127.re3. Sci STKE. 2002. PMID: 11943877 Review.
-
Communication between the TCR and integrins: role of the molecular adapter ADAP/Fyb/Slap.Curr Opin Immunol. 2002 Jun;14(3):317-22. doi: 10.1016/s0952-7915(02)00334-5. Curr Opin Immunol. 2002. PMID: 11973129 Review.
Cited by
-
A genome-wide in vivo CRISPR screen identifies essential regulators of T cell migration to the CNS in a multiple sclerosis model.Nat Neurosci. 2023 Oct;26(10):1713-1725. doi: 10.1038/s41593-023-01432-2. Epub 2023 Sep 14. Nat Neurosci. 2023. PMID: 37709997 Free PMC article.
-
Functional and Clinical Characterization of Tumor-Infiltrating T Cell Subpopulations in Hepatocellular Carcinoma.Front Genet. 2020 Sep 30;11:586415. doi: 10.3389/fgene.2020.586415. eCollection 2020. Front Genet. 2020. PMID: 33133170 Free PMC article.
-
Vav1 and mutant K-Ras synergize in the early development of pancreatic ductal adenocarcinoma in mice.Life Sci Alliance. 2020 Apr 10;3(5):e202000661. doi: 10.26508/lsa.202000661. Print 2020 May. Life Sci Alliance. 2020. PMID: 32277014 Free PMC article.
-
Molecular control of PDPNhi macrophage subset induction by ADAP as a host defense in sepsis.JCI Insight. 2025 Feb 4;10(6):e186456. doi: 10.1172/jci.insight.186456. JCI Insight. 2025. PMID: 39903516 Free PMC article.
References
-
- Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–133. - PubMed
-
- Avraham S, London R, Fu Y, Ota S, Hiregowdara D, Li J, Jiang S, Pasztor LM, White RA, Groopman JE, et al. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J Biol Chem. 1995;270:27742–27751. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

