TPC2 mediates new mechanisms of platelet dense granule membrane dynamics through regulation of Ca2+ release
- PMID: 26202466
- PMCID: PMC4569316
- DOI: 10.1091/mbc.E15-01-0058
TPC2 mediates new mechanisms of platelet dense granule membrane dynamics through regulation of Ca2+ release
Abstract
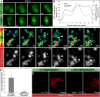
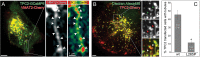
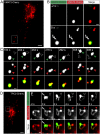
Platelet dense granules (PDGs) are acidic calcium stores essential for normal hemostasis. They develop from late endosomal compartments upon receiving PDG-specific proteins through vesicular trafficking, but their maturation process is not well understood. Here we show that two-pore channel 2 (TPC2) is a component of the PDG membrane that regulates PDG luminal pH and the pool of releasable Ca(2+). Using a genetically encoded Ca(2+) biosensor and a pore mutant TPC2, we establish the function of TPC2 in Ca(2+) release from PDGs and the formation of perigranular Ca(2+) nanodomains. For the first time, Ca(2+) spikes around PDGs--or any organelle of the endolysosome family--are visualized in real time and revealed to precisely mark organelle "kiss-and-run" events. Further, the presence of membranous tubules transiently connecting PDGs is revealed and shown to be dramatically enhanced by TPC2 in a mechanism that requires ion flux through TPC2. "Kiss-and-run" events and tubule connections mediate transfer of membrane proteins and luminal content between PDGs. The results show that PDGs use previously unknown mechanisms of membrane dynamics and content exchange that are regulated by TPC2.
© 2015 Ambrosio et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures
Similar articles
-
TPC2 controls pigmentation by regulating melanosome pH and size.Proc Natl Acad Sci U S A. 2016 May 17;113(20):5622-7. doi: 10.1073/pnas.1600108113. Epub 2016 May 2. Proc Natl Acad Sci U S A. 2016. PMID: 27140606 Free PMC article.
-
Acidic NAADP-releasable Ca(2+) compartments in the megakaryoblastic cell line MEG01.Biochim Biophys Acta. 2011 Aug;1813(8):1483-94. doi: 10.1016/j.bbamcr.2011.05.005. Epub 2011 May 13. Biochim Biophys Acta. 2011. PMID: 21601596
-
Characterization of Two-Pore Channel 2 by Nuclear Membrane Electrophysiology.Sci Rep. 2016 Feb 3;6:20282. doi: 10.1038/srep20282. Sci Rep. 2016. PMID: 26838264 Free PMC article.
-
Signal transduction and ion channels in guard cells.Philos Trans R Soc Lond B Biol Sci. 1998 Sep 29;353(1374):1475-88. doi: 10.1098/rstb.1998.0303. Philos Trans R Soc Lond B Biol Sci. 1998. PMID: 9800209 Free PMC article. Review.
-
Acidic Ca(2+) stores in platelets.Cell Calcium. 2011 Aug;50(2):168-74. doi: 10.1016/j.ceca.2010.11.011. Epub 2010 Dec 17. Cell Calcium. 2011. PMID: 21167599 Review.
Cited by
-
LAMTOR1 inhibition of TRPML1-dependent lysosomal calcium release regulates dendritic lysosome trafficking and hippocampal neuronal function.EMBO J. 2022 Mar 1;41(5):e108119. doi: 10.15252/embj.2021108119. Epub 2022 Jan 31. EMBO J. 2022. PMID: 35099830 Free PMC article.
-
Storage pool diseases illuminate platelet dense granule biogenesis.Platelets. 2017 Mar;28(2):138-146. doi: 10.1080/09537104.2016.1243789. Epub 2016 Nov 16. Platelets. 2017. PMID: 27849413 Free PMC article. Review.
-
Novel function of a dynein light chain in actin assembly during clathrin-mediated endocytosis.J Cell Biol. 2017 Aug 7;216(8):2565-2580. doi: 10.1083/jcb.201604123. Epub 2017 Jul 13. J Cell Biol. 2017. PMID: 28706108 Free PMC article.
-
Tetrandrine ameliorates cognitive deficits and mitigates tau aggregation in cell and animal models of tauopathies.J Biomed Sci. 2022 Oct 22;29(1):85. doi: 10.1186/s12929-022-00871-6. J Biomed Sci. 2022. PMID: 36273169 Free PMC article.
-
Human genome diversity data reveal that L564P is the predominant TPC2 variant and a prerequisite for the blond hair associated M484L gain-of-function effect.PLoS Genet. 2021 Jan 19;17(1):e1009236. doi: 10.1371/journal.pgen.1009236. eCollection 2021 Jan. PLoS Genet. 2021. PMID: 33465068 Free PMC article.
References
-
- Broos K, Feys HB, De Meyer SF, Vanhoorelbeke K, Deckmyn H. Platelets at work in primary hemostasis. Blood Rev. 2011;25:155–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

