The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study
- PMID: 26202488
- PMCID: PMC4656716
- DOI: 10.1007/s00198-015-3234-7
The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study
Abstract
The FREEDOM study and its Extension provide long-term information about the effects of denosumab for the treatment of postmenopausal osteoporosis. Treatment for up to 8 years was associated with persistent reduction of bone turnover, continued increases in bone mineral density, low fracture incidence, and a favorable benefit/risk profile.
Introduction: This study aims to report the results through year 5 of the FREEDOM Extension study, representing up to 8 years of continued denosumab treatment in postmenopausal women with osteoporosis.
Methods: Women who completed the 3-year FREEDOM study were eligible to enter the 7-year open-label FREEDOM Extension in which all participants are scheduled to receive denosumab, since placebo assignment was discontinued for ethical reasons. A total of 4550 women enrolled in the Extension (2343 long-term; 2207 cross-over). In this analysis, women in the long-term and cross-over groups received denosumab for up to 8 and 5 years, respectively.
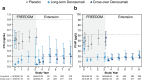
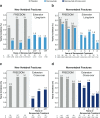
Results: Throughout the Extension, sustained reduction of bone turnover markers (BTMs) was observed in both groups. In the long-term group, mean bone mineral density (BMD) continued to increase significantly at each time point measured, for cumulative 8-year gains of 18.4 and 8.3 % at the lumbar spine and total hip, respectively. In the cross-over group, mean BMD increased significantly from the Extension baseline for 5-year cumulative gains of 13.1 and 6.2 % at the lumbar spine and total hip, respectively. The yearly incidence of new vertebral and nonvertebral fractures remained low in both groups. The incidence of adverse and serious adverse events did not increase over time. Through Extension year 5, eight events of osteonecrosis of the jaw and two events of atypical femoral fracture were confirmed.
Conclusions: Denosumab treatment for up to 8 years was associated with persistent reductions of BTMs, continued BMD gains, low fracture incidence, and a consistent safety profile.
Keywords: Bone mineral density; Clinical trial; Denosumab; Fracture; Osteoporosis; Safety.
Figures


Similar articles
-
Three-year denosumab treatment in postmenopausal Japanese women and men with osteoporosis: results from a 1-year open-label extension of the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT).Osteoporos Int. 2015 Feb;26(2):765-74. doi: 10.1007/s00198-014-2964-2. Epub 2014 Nov 18. Osteoporos Int. 2015. PMID: 25403903 Clinical Trial.
-
10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension.Lancet Diabetes Endocrinol. 2017 Jul;5(7):513-523. doi: 10.1016/S2213-8587(17)30138-9. Epub 2017 May 22. Lancet Diabetes Endocrinol. 2017. PMID: 28546097 Clinical Trial.
-
Further reductions in nonvertebral fracture rate with long-term denosumab treatment in the FREEDOM open-label extension and influence of hip bone mineral density after 3 years.Osteoporos Int. 2015 Dec;26(12):2763-71. doi: 10.1007/s00198-015-3179-x. Epub 2015 Jun 12. Osteoporos Int. 2015. PMID: 26068295 Free PMC article. Clinical Trial.
-
Effects of denosumab on bone mineral density and bone turnover in postmenopausal women.Pharmacotherapy. 2011 May;31(5):510-23. doi: 10.1592/phco.31.5.510. Pharmacotherapy. 2011. PMID: 21923432 Review.
-
Postmenopausal Osteoporosis: A Clinical Review.J Womens Health (Larchmt). 2018 Sep;27(9):1093-1096. doi: 10.1089/jwh.2017.6706. Epub 2018 Mar 27. J Womens Health (Larchmt). 2018. PMID: 29583083 Review.
Cited by
-
RANKL blockade for erosive hand osteoarthritis: a randomized placebo-controlled phase 2a trial.Nat Med. 2024 Mar;30(3):829-836. doi: 10.1038/s41591-024-02822-0. Epub 2024 Feb 15. Nat Med. 2024. PMID: 38361122 Free PMC article. Clinical Trial.
-
Denosumab-Associated Peri-Implant Atypical Femur Fracture: A Case Report.Hawaii J Health Soc Welf. 2019 Nov;78(11 Suppl 2):47-51. Hawaii J Health Soc Welf. 2019. PMID: 31773111 Free PMC article.
-
Spanish consensus on treat to target for osteoporosis.Osteoporos Int. 2018 Feb;29(2):489-499. doi: 10.1007/s00198-017-4310-y. Epub 2017 Nov 24. Osteoporos Int. 2018. PMID: 29177559 Free PMC article.
-
Efficacy and safety of Romosozumab in treatment for low bone mineral density: a systematic review and meta-analysis.Clin Rheumatol. 2020 Nov;39(11):3261-3276. doi: 10.1007/s10067-020-04948-1. Epub 2020 May 8. Clin Rheumatol. 2020. PMID: 32385757
-
Real-world data from a national survey on management of CKD-associated osteoporosis among Italian nephrologists.Arch Osteoporos. 2025 Jul 16;20(1):96. doi: 10.1007/s11657-025-01570-z. Arch Osteoporos. 2025. PMID: 40668510
References
-
- Papapoulos S, Chapurlat R, Libanati C, Brandi ML, Brown JP, Czerwinski E, Krieg MA, et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the freedom extension. J Bone Miner Res. 2012;27:694–701. doi: 10.1002/jbmr.1479. - DOI - PMC - PubMed
-
- Bone HG, Chapurlat R, Brandi ML, Brown JP, Czerwinski E, Krieg MA, Mellstrom D, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the freedom extension. J Clin Endocrinol Metab. 2013;98:4483–4492. doi: 10.1210/jc.2013-1597. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

