Synaptotagmins interact with APP and promote Aβ generation
- PMID: 26202512
- PMCID: PMC4511450
- DOI: 10.1186/s13024-015-0028-5
Synaptotagmins interact with APP and promote Aβ generation
Abstract
Background: Accumulation of the β-amyloid peptide (Aβ) is a major pathological hallmark of Alzheimer's disease (AD). Recent studies have shown that synaptic Aβ toxicity may directly impair synaptic function. However, proteins regulating Aβ generation at the synapse have not been characterized. Here, we sought to identify synaptic proteins that interact with the extracellular domain of APP and regulate Aβ generation.
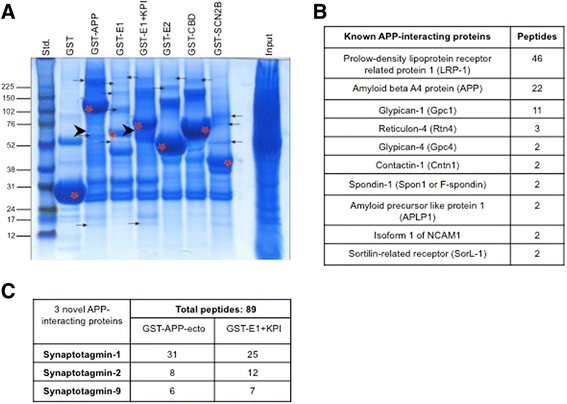
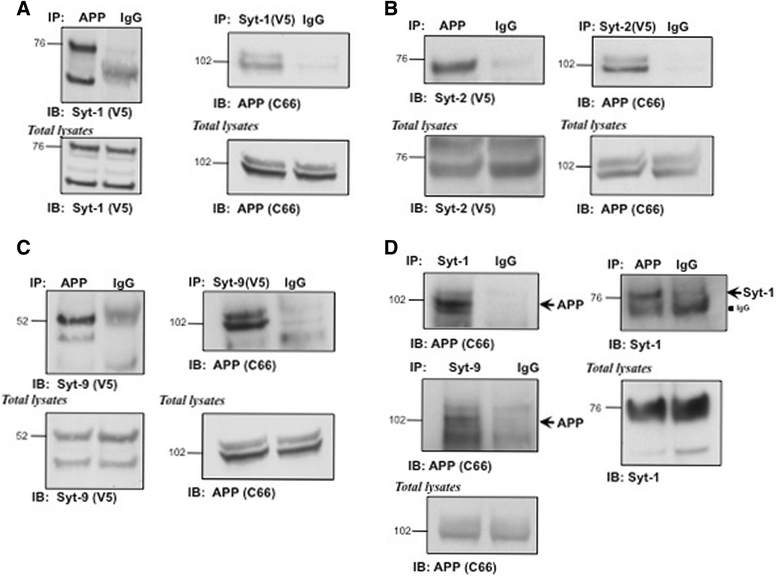
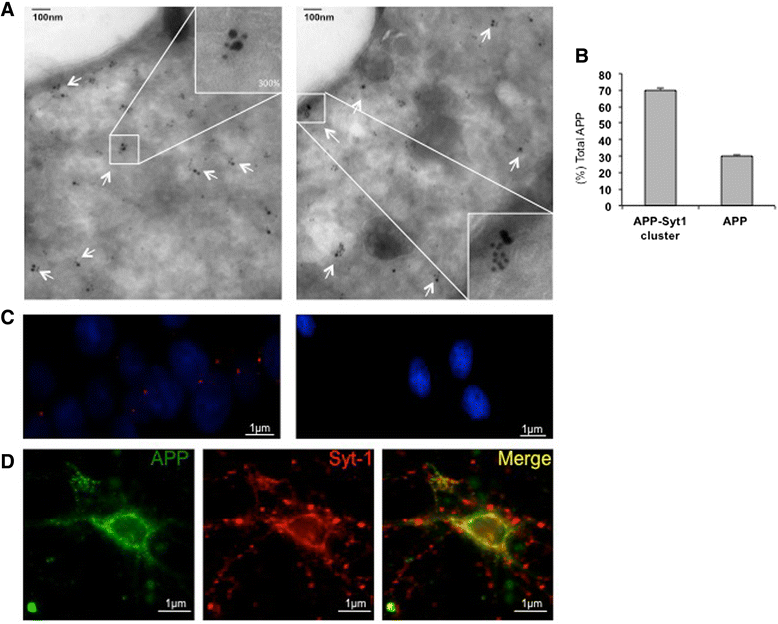
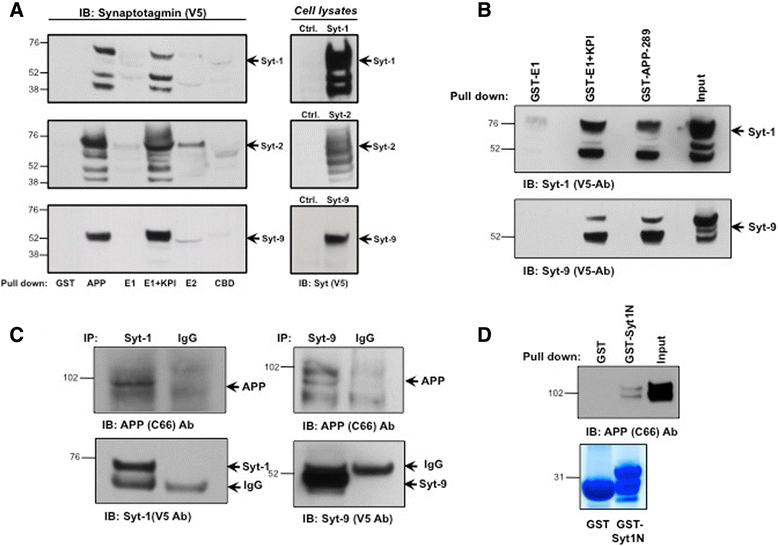
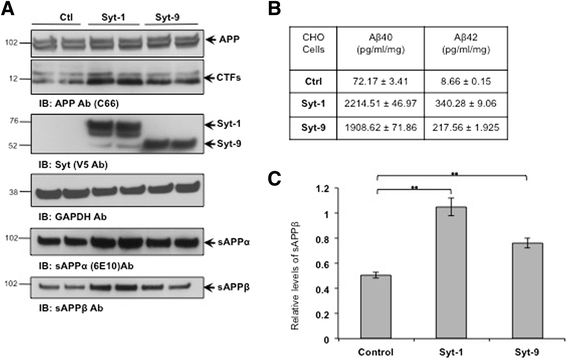
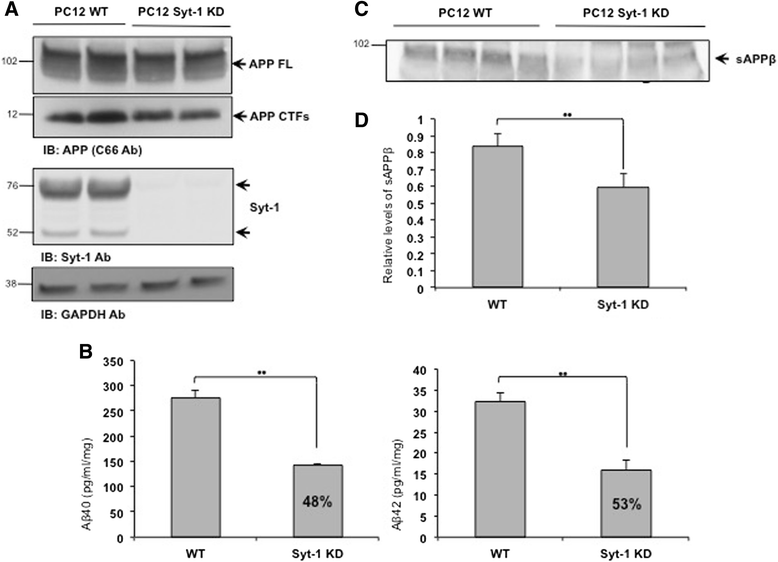
Results: Affinity purification-coupled mass spectrometry identified members of the Synaptotagmin (Syt) family as novel interacting proteins with the APP ectodomain in mouse brains. Syt-1, -2 and -9 interacted with APP in cells and in mouse brains in vivo. Using a GST pull-down approach, we have further demonstrated that the Syt interaction site lies in the 108 amino acids linker region between the E1 and KPI domains of APP. Stable overexpression of Syt-1 or Syt-9 with APP in CHO and rat pheochromocytoma cells (PC12) significantly increased APP-CTF and sAPP levels, with a 2 to 3 fold increase in secreted Aβ levels in PC12 cells. Moreover, using a stable knockdown approach to reduce the expression of endogenous Syt-1 in PC12 cells, we have observed a ~ 50% reduction in secreted Aβ generation. APP processing also decreased in these cells, shown by lower CTF levels. Lentiviral-mediated knock down of endogenous Syt-1 in mouse primary neurons also led to a significant reduction in both Aβ40 and Aβ42 generation. As secreted sAPPβ levels were significantly reduced in PC12 cells lacking Syt-1 expression, our results suggest that Syt-1 regulates Aβ generation by modulating BACE1-mediated cleavage of APP.
Conclusion: Altogether, our data identify the synaptic vesicle proteins Syt-1 and 9 as novel APP-interacting proteins that promote Aβ generation and thus may play an important role in the pathogenesis of AD.
Figures


Similar articles
-
Regulation of Synaptic Amyloid-β Generation through BACE1 Retrograde Transport in a Mouse Model of Alzheimer's Disease.J Neurosci. 2017 Mar 8;37(10):2639-2655. doi: 10.1523/JNEUROSCI.2851-16.2017. Epub 2017 Feb 3. J Neurosci. 2017. PMID: 28159908 Free PMC article.
-
Amyloid-β protein (Aβ) Glu11 is the major β-secretase site of β-site amyloid-β precursor protein-cleaving enzyme 1(BACE1), and shifting the cleavage site to Aβ Asp1 contributes to Alzheimer pathogenesis.Eur J Neurosci. 2013 Jun;37(12):1962-9. doi: 10.1111/ejn.12235. Eur J Neurosci. 2013. PMID: 23773065
-
Swedish mutant APP-based BACE1 binding site peptide reduces APP β-cleavage and cerebral Aβ levels in Alzheimer's mice.Sci Rep. 2015 Jun 19;5:11322. doi: 10.1038/srep11322. Sci Rep. 2015. PMID: 26091071 Free PMC article.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
The beta-secretase, BACE: a prime drug target for Alzheimer's disease.J Mol Neurosci. 2001 Oct;17(2):157-70. doi: 10.1385/JMN:17:2:157. J Mol Neurosci. 2001. PMID: 11816789 Review.
Cited by
-
Epigenetic modifications of DNA and RNA in Alzheimer's disease.Front Mol Neurosci. 2024 Apr 25;17:1398026. doi: 10.3389/fnmol.2024.1398026. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38726308 Free PMC article. Review.
-
Insights into the changes in the proteome of Alzheimer disease elucidated by a meta-analysis.Sci Data. 2021 Dec 3;8(1):312. doi: 10.1038/s41597-021-01090-8. Sci Data. 2021. PMID: 34862388 Free PMC article.
-
A superior loading control for the cellular thermal shift assay.Sci Rep. 2022 Apr 23;12(1):6672. doi: 10.1038/s41598-022-10653-7. Sci Rep. 2022. PMID: 35461337 Free PMC article.
-
Identification of the novel activity-driven interaction between synaptotagmin 1 and presenilin 1 links calcium, synapse, and amyloid beta.BMC Biol. 2016 Mar 31;14:25. doi: 10.1186/s12915-016-0248-3. BMC Biol. 2016. PMID: 27036734 Free PMC article.
-
Ensemble disease gene prediction by clinical sample-based networks.BMC Bioinformatics. 2020 Mar 11;21(Suppl 2):79. doi: 10.1186/s12859-020-3346-8. BMC Bioinformatics. 2020. PMID: 32164526 Free PMC article.
References
-
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261(13):6084–6089. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

