Maraviroc Pharmacokinetics in HIV-1-Infected Pregnant Women
- PMID: 26202768
- PMCID: PMC4614410
- DOI: 10.1093/cid/civ587
Maraviroc Pharmacokinetics in HIV-1-Infected Pregnant Women
Abstract
Objective: To describe the pharmacokinetics of maraviroc in human immunodeficiency virus (HIV)-infected women during pregnancy and post partum.
Methods: HIV-infected pregnant women receiving maraviroc as part of clinical care had intensive steady-state 12-hour pharmacokinetic profiles performed during the third trimester and ≥2 weeks after delivery. Cord blood samples and matching maternal blood samples were taken at delivery. The data were collected in 2 studies: P1026 (United States) and PANNA (Europe). Pharmacokinetic parameters were calculated.
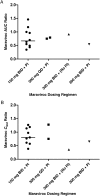
Results: Eighteen women were included in the analysis. Most women (12; 67%) received 150 mg of maraviroc twice daily with a protease inhibitor, 2 (11%) received 300 mg twice daily without a protease inhibitor, and 4 (22%) had an alternative regimen. The geometric mean ratios for third-trimester versus postpartum maraviroc were 0.72 (90% confidence interval, .60-.88) for the area under the curve over a dosing interval (AUCtau) and 0.70 (0.58-0.85) for the maximum maraviroc concentration. Only 1 patient showed a trough concentration (Ctrough) below the suggested target of 50 ng/mL, both during pregnancy and post partum. The median ratio of maraviroc cord blood to maternal blood was 0.33 (range, 0.03-0.56). The viral load close to delivery was <50 copies/mL in 13 women (76%). All children were HIV negative at testing.
Conclusions: Overall maraviroc exposure during pregnancy was decreased, with a reduction in AUCtau and maximum concentration of about 30%. Ctrough was reduced by 15% but exceeded the minimum Ctrough target concentration. Therefore, the standard adult dose seems sufficient in pregnancy.
Clinical trials registration: NCT00825929 and NCT000422890.
Trial registration: ClinicalTrials.gov NCT00422890 NCT00825929 NCT00422890 NCT00825929.
Keywords: HIV; MTCT; maraviroc; pharmacokinetics; pregnancy.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- UNAIDS. AIDS epidemic update, 2013. Available at: http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/... Accessed 22 January 2015.
-
- UNAIDS. The GAP report, 2013, Available at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_... Accessed 22 January 2015.
-
- Harris NS, Fowler MG, Sansom SL, Ruffo N, Lampe MA. Use of enhanced perinatal human immunodeficiency virus surveillance methods to assess antiretroviral use and perinatal human immunodeficiency virus transmission in the United States, 1999–2001. Am J Obstet Gynecol 2007; 197:S33–41. - PubMed
-
- MacArthur RD, Novak RM. Reviews of anti-infective agents: maraviroc: the first of a new class of antiretroviral agents. Clin Infect Dis 2008; 47:236–41. - PubMed
-
- Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents, 2014. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf Accessed 2 September 2014.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

